More Information
Submitted: February 19, 2025 | Approved: March 04, 2025 | Published: March 05, 2025
How to cite this article: Rai V, Kumar P. Detrimental Effects of Methylenetetrahydrofolate Reductase (MTHFR) Gene Polymorphism on Human Reproductive Health: A Review. Clin J Obstet Gynecol. 2025; 8(1): 007-014. Available from:
https://dx.doi.org/10.29328/journal.cjog.1001182
DOI: 10.29328/journal.cjog.1001182
Copyright license: © 2025 Rai V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: MTHFR; Folate; Homocysteine; Male infertility; Preeclampsia; PCOS; Congenital anomalies
Detrimental Effects of Methylenetetrahydrofolate Reductase (MTHFR) Gene Polymorphism on Human Reproductive Health: A Review
Vandana Rai* and Pradeep Kumar
Human Molecular Genetics Laboratory, Department of Biotechnology, V B S Purvanchal University, Jaunpur-222003, India
*Address for Correspondence: Vandana Rai, Human Molecular Genetics Laboratory, Department of Biotechnology, V B S Purvanchal University, Jaunpur-222003, India, Email: [email protected]
Methylenetetrahydrofolate Reductase (MTHFR) is an important enzyme of the folate cycle, which is required to convert 5,10-methyltetrahydrofolate into 5-methyltetrahydrofolate (5-methylTHHF). 5-methyl THF is a methyl group donor for several cellular methylation processes. It also donates methyl group for the conversion of homocysteine into methionine, the higher concentration of which is toxic. MTHFR gene C677T polymorphism is clinically important polymorphism and the variant MTHFR (A222V) enzyme has reduced activity, hence increasing the requirement for folic acid. Less conversion of folate to 5-methyl-THF due to C677T polymorphism results in a higher plasma concentration of homocysteine (hyperhomocysteinemia). Individuals having C677T polymorphism are susceptible to various diseases, including reproductive problems like male infertility, polycystic ovary syndrome, Recurrent Pregnancy Loss (RPL), Preeclampsia (PE), placental abruption, and adverse pregnancy outcomes. MTHFR C677T polymorphism mimics folate deficiency, and folate is required for DNA synthesis, repair, methylation, and proper chromosome segregation, and all these processes are important for foetal growth and normal development. Methylation and demethylation processes control the gene expression of about 45% of human genes. Impaired methylation influences the expression of genes involved in the regulation of hormones, spermatogenesis, and oogenesis. In males, oxidative stress damages sperm DNA decreases sperm motility, and may impair fertilization capability. In pregnant women, hyperhomocysteinemia increases oxidative stress and inflammation within the placenta, which causes damage to placental tissue, impairs its function, and disrupts foetal development. Further, hyperhomocysteinemia (HHcy) is embryotoxic and neurotoxic and is responsible for congenital anomalies in the foetus. This review supports the idea that MTHFR C677T polymorphism is associated with an increased risk for male infertility, PCOS, RPL, PE, and congenital anomalies. This review may provide a clue toward a better understanding of the correlation between the MTHFR C677T polymorphism and its detrimental effects on human reproductive health.
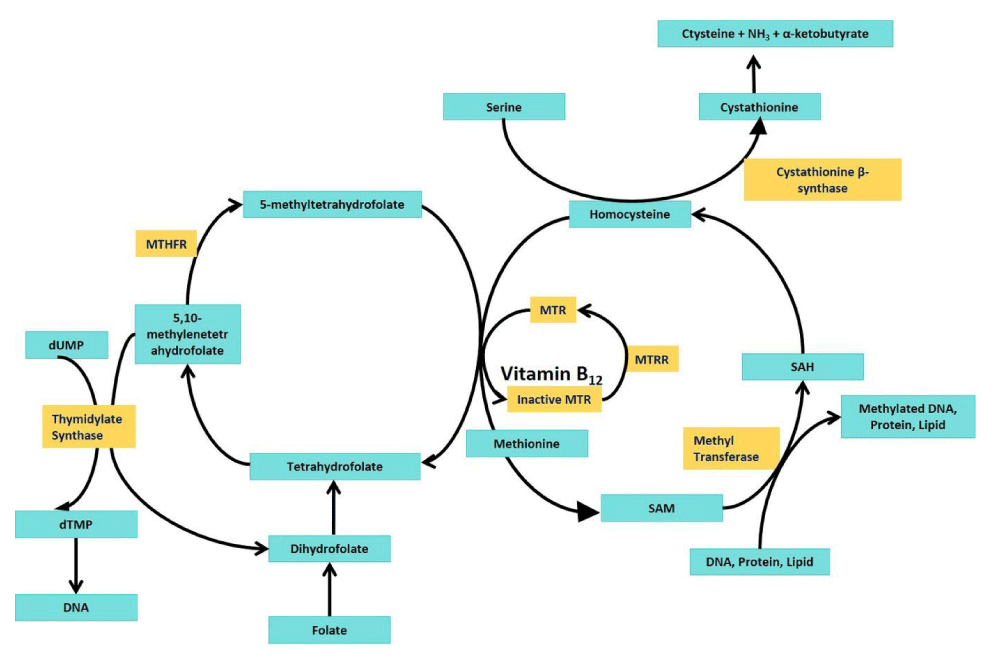
The folate cycle is essential for cellular functions such as DNA synthesis, DNA repair, cellular methylation, and amino acid metabolism. It involves the conversion of folate into various forms, including tetrahydrofolate (THF) and its derivatives, which are required for key biochemical reactions. The methylenetetrahydrofolate reductase (MTHFR) enzyme plays a critical role in folate (vitamin B9) processing. The enzyme MTHFR plays a key role in converting 5,10-methylene-THF (a form of folate) into 5-methyl-THF and regulates the levels of homocysteine, a potentially harmful sulphur-containing amino acid. 5-methyl-THF is used to donate a methyl group for the conversion of homocysteine into methionine in the methionine cycle. Folate and methionine cycles are two metabolic pathways that work together and are linked by the metabolite 5-methyl-THF. S-adenosyl methionine (SAM) is produced from methionine and is the primary methyl donor in many methylation reactions, including the methylation of DNA, protein, and lipid (Figure 1). This is a vital process for regulating gene expression, normal chromosome segregation, and maintaining normal cell function. MTHFR enzyme is a homotetramer; each subunit is roughly 77 kDa in size, and the weight of the tetrameric enzyme is about 308 kDa. The active site of MTHFR contains the binding site for 5,10-methylene-THF and NADPH, which is required for the reduction reaction. The enzyme also requires vitamin B12 (riboflavin) for its proper function [1].
Figure 1: Folate-methionine cycle: MTHFR- methylenetetrahysrofolat; MTR- Methionine synthase; MTRR- Methionine synthase reductase; SAM-S-adenosylmethionine; SAH- S-adenosylhomocysteine; dUMP-deoxyuridinemonophsphate; dTMP- deoxyuridinemonophsphate.
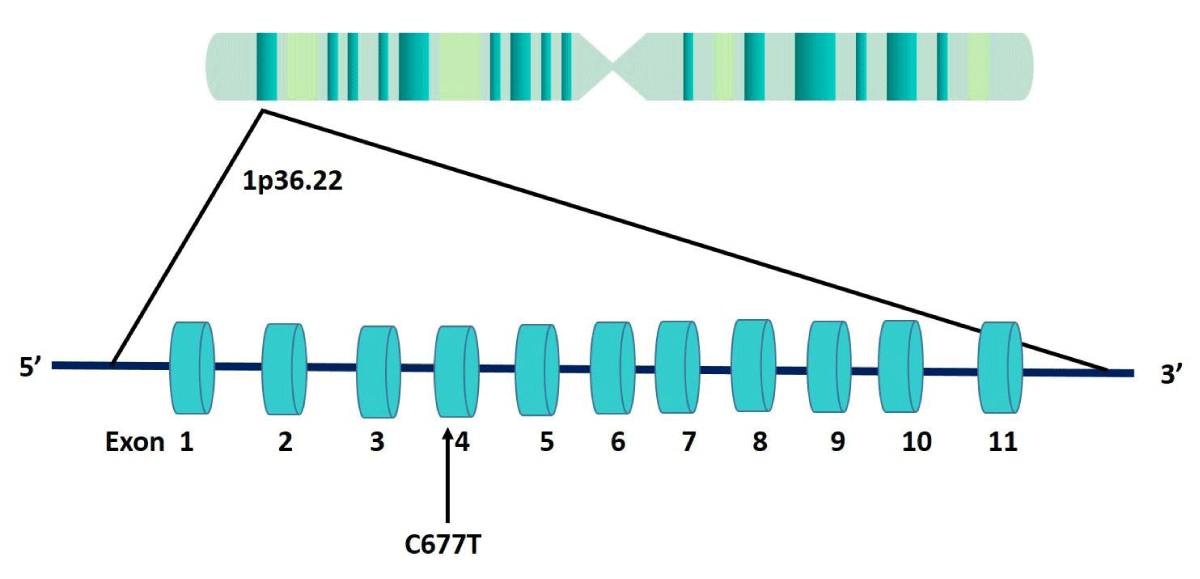
The MTHFR gene encodes the enzyme MTHFR. Gene is located on chromosome 1, at position p36.3 region, consists of 11 exons, and spans about 24 kb of DNA. Several polymorphisms are reported in the MTHFR gene, but the C677T polymorphism (rs 1801133) is one of the most studied genetic variants due to its impact on folate metabolism. In C677T polymorphism, cytosine (C) is replaced by thymine (T) at position 677 in exon 4, and this substitution causes a change in the amino acid sequence of the MTHFR enzyme (Ala222Val) (Figure 2). The variant MTHFR enzyme (222Val) is thermolabile and exhibits reduced activity compared with the wild type, making it less efficient. C677T polymorphism reduces the enzymatic activity of the MTHFR enzyme, and the variant MTHFR enzyme can impair the conversion of homocysteine to methionine, leading to elevated homocysteine levels in the blood [2,3].
Figure 2: MTHFR gene structure showing the position of C677T polymorphism in exon 4.
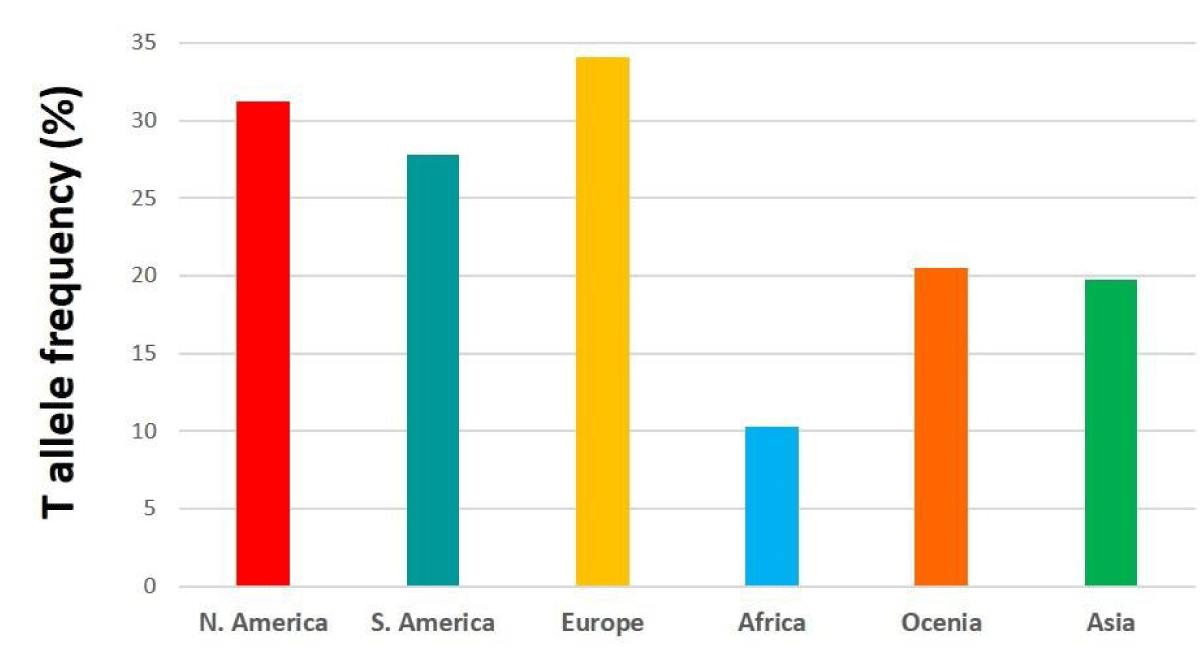
Figure 3: Bar diagram showing T allele frequency in different continents.
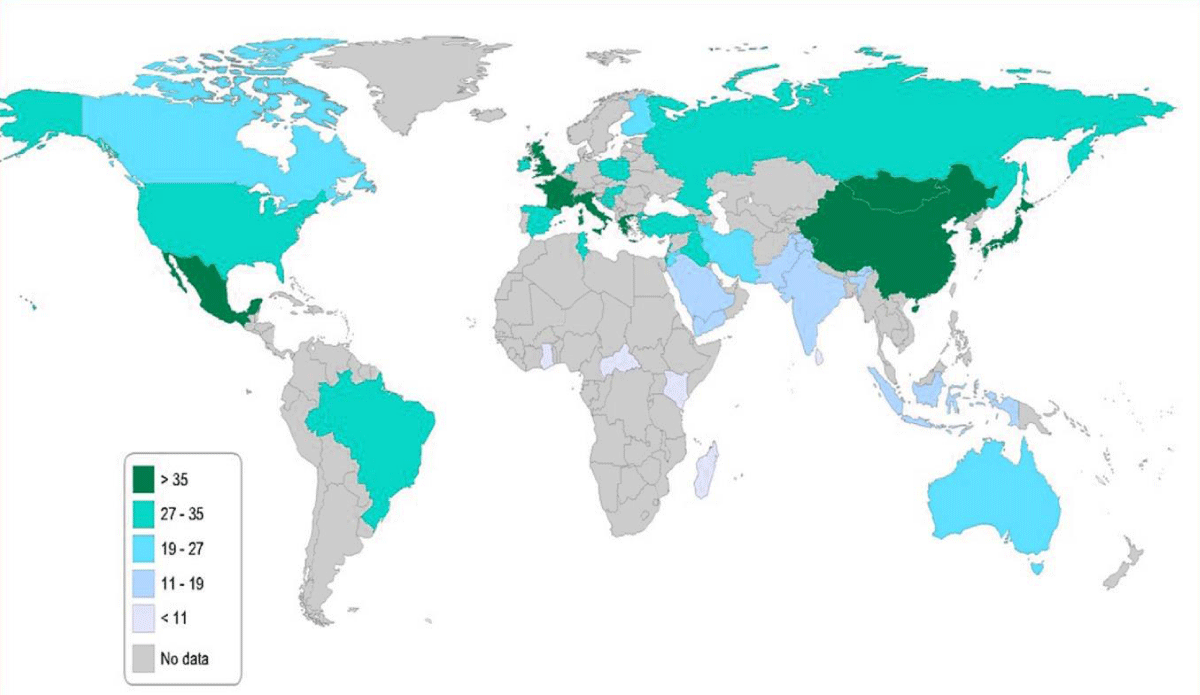
The global frequency of this polymorphism varies significantly by population [4-9]. (The frequency of the T allele in North America, South America, Europe, Africa, Oceania, and Asia was reported as 31.2%, 27.8%, 34.1%, 10.3%, 20.5%, and 19.7% respectively (Figure 3) [9]. The highest frequency of the T allele is reported in Mexico and China (> 35%) (Figure 4).
Figure 4: Map showing T allele frequency in different countries of the world.
The detrimental effects of the mutation on the enzymatic activity of MTHFR depend on the status of the folate. The mutation might be tolerated in subjects with a rich folate supply, whereas in individuals with folate insufficiency/deficiency, it might result in some biochemical or clinical phenotypic consequences [3,10]. Reduced MTHFR activity results in an increased requirement for folic acid to maintain normal homocysteine remethylation.
Individuals with the MTHFR gene C677T polymorphism may be at an increased risk of congenital defects [11-13], metabolic disorders [14], psychiatric disorders [15,16], neurological disorders [17,18], and cancer [19-22]. There is several evidence suggesting that the C677T polymorphism may be associated with reproductive health problems such as male infertility, polycystic ovary syndrome, preeclampsia, Recurrent Pregnancy Loss/ miscarriage (RPL), placental abruption, Preterm Birth (PTB), Intrauterine Foetal Death (IUFD), and congenital anomalies, etc. Hence, in the present review, authors have tried to summarize the detrimental effects caused by the C677T polymorphism on reproductive health.
MTHFR C677T polymorphism and male infertility
Male infertility is a global health concern, affecting 40% - 50% of couples worldwide [23]. It is a multifactorial clinical disorder with genetic as well as environmental causes. Several genetic risk factors for male infertility have been reported, like aneuploidies, chromosomal translocation, chromosome microdeletions, and gene mutations. About 50% of cases of male infertility are idiopathic. Some common causes of male infertility include: low sperm count, poor sperm motility, abnormal sperm morphology, hormonal imbalances, varicocele, infections, ejaculation issues, environmental factors, and genetic factors.
Spermatogenesis is a very complex event that is controlled by the expression of several genes. Methylation and demethylation play important roles in the production of germ cells. The correct methylation of DNA ensures appropriate chromatin concentration in the sperm head, enabling sperm maturation and its ability in fertilization and post-fertilization events [24]. Numerous studies reported C677T polymorphism as a risk factor for male infertility [10,25-28]. The MTHFR gene polymorphism affects the folate cycle and one-carbon cycle, leading to Hcy accumulation, abnormal DNA methylation, and decreased antioxidant capacity, ultimately affecting sperm quality and causing male infertility [29].
C677T polymorphism increases homocysteine concentrations in blood (hyperhomocysteinemia (HHcy)), which is reported to be an important risk factor for cardiovascular diseases. HHcy damage to endothelial cells may interfere with penile blood supply or other erection mechanisms [30], leading to ejaculation problems [31,32]. Oxidative stress plays an important role in male infertility [33] and Atig and colleagues [34] reported that about 30% - 80% of male infertility cases are due to Reactive Oxygen Species (ROS)-induced higher oxidative stress. Sperms are particularly intolerant to oxidative stress because, during spermatogenesis, they lose their DNA repair ability. Several studies confirmed that antioxidant supplementation improves semen parameters [35]. Oxidative stress causes lipid peroxidation (peroxidation of polyunsaturated fatty acids of sperm membrane), DNA fragmentation (oxidative modifications), oxidative modifications of proteins, and mitochondrial damage in sperms [36-39]. Oxidative stress can lead to DNA strand breaks and cause genetic mutations, lead to developmental defects in embryos, and a heightened miscarriage risk [38].
Individuals with the T allele have reduced MTHFR activity, so the reduced availability of methylfolate could impair spermatogenesis, leading to sperm DNA fragmentation and poor sperm quality. The C677T polymorphism could influence male fertility in several ways: (i) C677T is associated with higher levels of homocysteine, which can lead to oxidative stress, oxidative stress damages sperm DNA, decreases sperm motility, and may impair fertilization capability [40], (ii) folate-dependent methylation processes are disrupted, which are critical for spermatogenesis. Supplementation with vitamins B6, B12, and folic acid might improve homocysteine metabolism and reduce oxidative damage, potentially improving sperm function, motility, and viability. Supplementation with antioxidants (e.g., vitamins C, E, and zinc) may help reduce oxidative stress and improve sperm motility and viability.
MTHFR C677T polymorphism and polycystic ovary syndrome (PCOS)
PCOS, also known as polycystic ovary disease (PCOD), is a heterogeneous condition associated with endocrinopathy. PCOS is associated with an increased risk of pregnancy loss with a prevalence of approximately 2% - 25% of women of reproductive age (18 years - 44 years). Stress, obesity, hormonal fluctuations, lifestyle, and genetics are the major risk factors for PCOS. About 70% of PCOS females are infertile [41]. Besides ovarian cysts, several other conditions are also associated with PCOS, like dyslipidaemia, hypertension, type 2 Diabetes, hepatic steatosis, hyperandrogenism, hirsutism, depression, anxiety, etc. It may also cause endometrial carcinoma. PCOS is a polygenic and multifactorial disorder. Cooper and colleagues [42] first reported the genetic basis of PCOS. Since then, candidate gene variants are reported as risk factors for PCOS susceptibility, such as – CYP11a, CYP17, CYP19, CYP21, PCOS1, FTO, AR, LH, FSHR, INS, IRS1, IRS2, NCOR1, PPARG1, and MTHFR, etc.
Hyperhomocysteinemia (HHcy) in PCOS patients is frequently reported, and MTHFR polymorphism is an important genetic cause for elevated homocysteine concentration, several experimental and clinical pieces of evidence suggested that disturbances in the folate-methionine cycle resulted in ovarian dysfunctions. First of all, Glueck and colleagues [43] have reported an association between the MTHFR C677T polymorphism and PCOS. After that, numerous studies reported C677T polymorphism as a risk factor for PCOS [44,45]. In a recent meta-analysis, authors reported a significant association between the T allele and PCOS risk (OR = 1.31; 95% CI 1.07 -1.62; p = 0.008) [46]. The C677T polymorphism leads to reduced activity of the MTHFR enzyme, which results in an impaired ability to process folate and homocysteine.
In summary, MTHFR C677T Might contribute to PCOS by several mechanisms, like-
- Folate is essential for DNA synthesis, repair, and methylation and a hypoactive MTHFR affects cell growth and division, which is crucial for reproductive tissues, including the ovaries.
- Impaired methylation influences the expression of genes involved in the regulation of hormones and ovarian function, potentially exacerbating conditions, like PCOS.
- Elevated homocysteine concentration contributes to insulin resistance, a hallmark of PCOS, and endothelial dysfunction, which could worsen metabolic issues related to PCOS.
- Hyperhomocysteinemia causes vascular dysfunction, increased androgen production, and reproductive issues.
- Elevated homocysteine levels also contribute to inflammation and oxidative damage in various tissues, including the ovaries, and so worsen the metabolic and reproductive disturbances in PCOS.
MTHFR C677T polymorphism and recurrent pregnancy loss (RPL)/spontaneous abortion (SA) /miscarriage
Recurrent Pregnancy Loss (RPL) is defined as having two or more miscarriages. Risk factors for RPL include uterine problems, hormonal disorders, autoimmune diseases (such as antiphospholipid antibody syndrome), lifestyle and environmental factors, and genetic abnormalities. Most miscarriages that are unexplained, that is idiopathic, are thought to be inherited. The most common genetic factors contributing to miscarriage are aneuploidy, chromosomal abnormalities in the embryo (translocations), and the presence of candidate gene variants in the mother.
Candidate genes associated with RPL include KIF14, CEP55, STIL, FOXP3, GLE, RYR1, POMT1, DYNC2H1, ALOX15, MMP9, MMP10, TNC, FKBP4, ATAMTS1, FOXA2, FGA, F13A1, KHDC3L, VEGF, ESR, and MTHFR. These genes are often implicated in issues related to cell division, foetal movement, angiogenesis, immune response, and inflammation, which are critical for successful pregnancy development. Numerous studies suggest that women with the MTHFR gene polymorphism may have an increased risk of recurrent pregnancy loss [47-51]. However, some other studies have failed to establish a clear, direct link between MTHFR mutations and miscarriage risk [52-54]. Wen and colleagues [55] have reported a significant association between the C677T polymorphism and spontaneous abortion (OR = 1.43; 95% CI = 1.25 - 1.64, p < 0.01).
Reduced MTHFR enzyme activity leads to higher levels of homocysteine in the blood, i.e. hyperhomocysteinemia. Elevated homocysteine levels can cause problems like impaired placental blood flow, increased clotting risk, and inflammation, all of which can interfere with a healthy pregnancy. Higher homocysteine levels can have several negative effects on pregnancy:
- Homocysteine has prothrombotic effects; it can increase the likelihood of blood clot formation, and blood clots in the placental vessels can obstruct the blood supply to the fetus, increasing the risk of miscarriage or stillbirth.
- Elevated homocysteine levels can increase oxidative stress and inflammation within the placenta, which causes damage to placental tissue, impairs its function, and disrupts foetal development.
- Homocysteine damages blood vessels and affects the endothelial lining of the blood vessels, which impairs placental blood flow, which is critical for supplying oxygen and nutrients to the developing fetus.
- High homocysteine levels impair ovum quality and lead to difficulties with conception and early pregnancy loss.
The MTHFR mutation may impair the ability to metabolize folate, but supplementation with folate or its active form (5-methyltetrahydrofolatefolate), vitamins B6 and B12, can help lower homocysteine levels and mitigate the negative effects of hyperhomocysteinemia.
MTHFR C677T polymorphism and preeclampsia
The MTHFR C677T polymorphism has been studied for its potential role in preeclampsia (PE) development due to its impact on homocysteine metabolism and folate processing. PE is a pregnancy-related condition characterised by high blood pressure (systolic blood pressure >140 mmHg and diastolic blood pressure >90 mmHg) and proteinuria (>300 mg/day). The prevalence of PE is approximately 2% - 5%, causing the death of 40,000 pregnant women per annum globally [56]. PE complications start during the second and third trimesters of pregnancy and are life-threatening for both the mother and baby if not properly managed. If PE remains untreated, there is a risk of organ damage, such as the kidneys, often after the 20th week of pregnancy. PE involves the release of angiogenic factors, oxidative stress, hyperlipidaemia, and increased insulin resistance, resulting in sustained inflammation and endothelial dysfunction [57,58]. Several studies have reported MTHFR C677T polymorphism as a potential risk factor for PE [59,60]. Reduced activity of variant MTHFR enzyme reduces the conversion of homocysteine to methionine; hence the homocysteine level is increased. Elevated homocysteine levels are associated with an increased risk of cardiovascular diseases and thrombotic events, which can contribute to the development of preeclampsia by affecting the blood vessels [61]. Elevated homocysteine levels are known to promote oxidative stress, inflammation, and damage to the endothelium (the inner lining of blood vessels). This vascular dysfunction can increase the risk of developing hypertension and preeclampsia. Homocysteine may also interfere with the production of nitric oxide, which is vital for blood vessel dilation and regulating blood pressure.
Placental dysfunction due to folate deficiency and hyperhomocysteinemia is a key feature in the development of preeclampsia. Impaired trophoblastic invasion and poor placental remodelling can lead to reduced blood flow and oxygen delivery to the placenta, resulting in preeclampsia. In the case of preeclampsia, the placenta is unable to supply adequate nutrients and oxygen to the foetus.
Individuals with a single copy of the T allele (heterozygous condition) have mild MTHFR enzyme activity reduction. So the risk of preeclampsia may be slightly higher compared to those with the normal CC genotype. However, women with the TT genotype (mutant homozygous) have more pronounced MTHFR dysfunction, which could potentially increase their risk for preeclampsia, especially if combined with environmental factors such as low folate intake or pre-existing vascular conditions.
The MTHFR C677T polymorphism may cause preeclampsia through its effect on homocysteine levels, which can lead to (i) vascular dysfunction, (ii) reduced production of nitric oxide, (iii) oxidative stress, and (iv) placental insufficiency.
MTHFR C677T polymorphism and adverse pregnancy outcomes
Adverse pregnancy outcomes, including Preterm Birth (PTB), Low Birth Weight (LBW), small-for-gestational-age (SGA), and congenital anomalies, are major determinants for infant morbidity and mortality. PTB and its complications are the leading causes of death among children under 5 years old [62]. Environmental and genetic factors play an important role in the occurrence of PTB, LBW, and SGA [63-66].
Candidate gene studies for PTB and LBW are very few, and the results are inconsistent [67-69]. Two studies, one from India and the other from Japan suggested that the folic acid metabolic enzyme MTHFR C677T polymorphism is a risk for PTB, LBW, and SGA [70]. Kramer, et al. [71] showed that the lower level of folate and hyperhomocysteinaemia contributes to adverse pregnancy outcomes.
The MTHFR C677T polymorphism has been associated with an increased risk of preterm birth [72,73]. This may be related to the effects of elevated homocysteine on placental development and function. Hyperomocysteinemia causes placental abruption, a condition where the placenta detaches prematurely from the uterine wall leading to early delivery in some cases. This can lead to severe bleeding and complications for both the mother and the baby and results in preterm birth or miscarriage. Elevated homocysteine levels damage blood vessels, increase blood clotting, oxidative stress, and inflammation may trigger premature labour [72,73].
The maternal and foetal MTHFR C677T polymorphism has been reported as a risk factor for congenital anomalies such as Neural Tube Defects (NTD) [13], cleft lip and palate [12], and Down syndrome [11], etc. Higher concentrations of homocysteine are teratogenic in the first and mid-trimester [74]. Several evidence suggest that elevated homocysteine levels may interfere with craniofacial development, heart and kidney, etc. Other factors, such as maternal folate status, environmental influences, and additional genetic variations, likely also play a role in the development of congenital anomalies. Mothers with the TT genotype variant (mutant homozygous) are generally at higher risk of DNA hypomethylation, abnormal gene expression, and elevated homocysteine levels and have a higher risk of having a foetus with congenital anomalies. It has been established that the higher concentrations of homocysteine have embryotoxic and neurotoxic effects. It impairs DNA repair in hippocampal neurones and promotes apoptosis [75]. MTHFR polymorphism/folate deficiency and/or hyperhomocysteinemia act prenatally and affect the central nervous system of the foetus through different mechanisms such as (i) folate is required for nucleotide synthesis; its deficiency impairs neural progenitor division and neuron migration, (ii) folate deficiency affects neurogenesis, and (iii) impaired methylation results in abnormal gene expression. Women with the C677T polymorphism should be encouraged to take higher doses of folate, especially in the periconceptional period and early pregnancy. This can help reduce the risk of congenital anomalies and possibly reduce the risk of miscarriage and other adverse pregnancy outcomes.
The MTHFR C677T polymorphism is associated with several detrimental effects on reproductive health, including male infertility, PCOS, miscarriage, preeclampsia, preterm birth, placental abruption, and congenital anomalies. These risks are often related to impaired folate metabolism and elevated homocysteine levels. Management through folate and antioxidants (Vitamins C, E, and Zinc) supplementation, monitoring of homocysteine levels, and genetic counselling can help mitigate these risks and improve reproductive health outcomes. The takeaway message is that every woman and man of fertile age should get their MTHFR gene checked before pregnancy planning for children for the C677T polymorphism, and if the T allele is present, then they should increase the intake of folate, especially women to avoid recurrent pregnancy loss, preeclampsia, low weight birth, preterm birth, and congenital anomalies, etc.
- Gos M, Szpecht-Potoka A. Genetic basis of neural tube defects. II. Genes correlated with folate and methionine metabolism. J Appl Genet. 2002;5:511-524. Available from: https://pubmed.ncbi.nlm.nih.gov/12441636/
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111-113. Available from: https://pubmed.ncbi.nlm.nih.gov/7647779/
- Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7-9. Available from: https://doi.org/10.1161/01.cir.93.1.7
- Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet. 1998;62:1258-1260. Available from: https://doi.org/10.1086/301836
- Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M, et al. Geographical and ethnic variation of the 677CT allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas worldwide. J Med Genet. 2003;40:619-625. Available from: https://doi.org/10.1136/jmg.40.8.619
- Spiridonova MG, Stepanov VA, Maksimova NR, Puzyrev VP. Population study of frequency of methylenetetrahydrofolate reductase C677T gene polymorphism in Yakutia. Genetika. 2004;40:704-708. Available from: https://pubmed.ncbi.nlm.nih.gov/15272569/
- Rai V, Yadav U, Kumar P, Yadav SK. Methylenetetrahydrofolate reductase polymorphism (C677T) in Muslim population of Eastern Uttar Pradesh, India. Indian J Med Sci. 2010;64(5):219-223. Available from: https://pubmed.ncbi.nlm.nih.gov/22842321/
- Rai V, Yadav U, Kumar P. Genotype prevalence and allele frequencies of 5, 10-methylenetetrahydrofolate reductase (MTHFR) C677T mutation in two caste groups of India. Cell Mol Biol. 2012;58:OL1695-1701. Available from: https://www.cellmolbiol.org/index.php/CMB/article/view/553/226
- Yadav U, Kumar P, Gupta S, Rai V. Distribution of MTHFR C677T gene polymorphism in healthy North Indian population and an updated meta-analysis. Ind J Clin Biochem. 2018;32(4):399-410. Available from: https://doi.org/10.1007/s12291-016-0619-0
- A ZC, Yang Y, Zhang SZ, Li N, Zhang W. Single nucleotide polymorphism C677T in the methylenetetrahydrofolate reductase gene might be a genetic risk factor for infertility in Chinese men with azoospermia or severe oligozoospermia. Asian J Androl. 2007;9:57-62. Available from: https://doi.org/10.1111/j.1745-7262.2007.00225.x
- Rai V, Yadav U, Kumar P, Yadav SK, Mishra OP. Maternal methylenetetrahydrofolate reductase C677T polymorphism and Down syndrome risk: A meta-analysis from 34 studies. PLoS One. 2014;9(9):e108552. Available from: https://doi.org/10.1371/journal.pone.0108552
- Rai V. Strong association of C677T polymorphism of methylenetetrahydrofolate reductase gene with nonsyndromic cleft lip/palate (nsCL/P). Ind J Clin Biochem. 2017;33(1):5-15. Available from: https://doi.org/10.1007/s12291-017-0673-2
- Yadav U, Kumar P, Yadav SK, Mishra OP, Rai V. Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: An updated meta-analysis. Metab Brain Dis. 2015;30:7-24. Available from: https://doi.org/10.1007/s11011-014-9575-7
- Rai V. The MTHFR C677T polymorphism and hyperuricemia risk: A meta-analysis of 558 cases and 912 controls. Metabolomics. 2016;6:166. Available from: http://dx.doi.org/10.4172/2153-0769.1000166
- Rai V. Evaluation of methylenetetrahydrofolate reductase gene variant (C677T) as risk factor for bipolar disorder. Cell Mol Biol. 2011;57:1558-1566. Available from: https://pubmed.ncbi.nlm.nih.gov/21955385/
- Yadav U, Kumar P, Gupta S, Rai V. Role of MTHFR C677T gene polymorphism in the susceptibility of schizophrenia: An updated meta-analysis. Asian J Psychiatry. 2016;20:41-51. Available from: https://doi.org/10.1016/j.ajp.2016.02.002
- Rai V, Kumar P. Methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to epilepsy. Neurol Sci. 2018;39:2033-2041. Available from: https://doi.org/10.1007/s10072-018-3583-z
- Rai V, Kumar P. Relation between methylenetetrahydrofolate reductase polymorphisms (C677T and A1298C) and migraine susceptibility. Ind J Clin Biochem. 2017;37:3-17. Available from: https://doi.org/10.1007/s12291-021-01000-0
- Rai V. Folate pathway gene MTHFR C677T polymorphism and risk of lung cancer in Asian populations. Asian Pac J Cancer Prev. 2014;15(21):9259-9264. Available from: https://doi.org/10.7314/apjcp.2014.15.21.9259
- Kumar P, Yadav U, Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and breast cancer risk: Evidence for genetic susceptibility. MetaGene. 2015;6:72-84. Available from: https://doi.org/10.1016/j.mgene.2015.08.008
- Kumar P, Rai V. Methylenetetrahydrofolate reductase C677T polymorphism and risk of esophageal cancer: An updated meta-analysis. Egypt J Med Hum Genet. 2018;19(4):273-284. Available from: https://www.ajol.info/index.php/ejhg/article/view/181774
- Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and its association with ovary cancer. J Health Med Inform. 2016;7:3. Available from: https://www.hilarispublisher.com/open-access/methylenetetrahydrofolate-reductase-gene-c677t-polymorphism-and-itsassociation-with-ovary-cancer-2157-7420-1000226.pdf
- Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci. 2015;8:191-196. Available from: https://doi.org/10.4103/0974-1208.170370
- Carrell DT. The sperm epigenome: Implications for assisted reproductive technologies. Adv Exp Med Biol. 2019;1166:47-56. Available from: https://doi.org/10.1007/978-3-030-21664-1_3
- Pei J. Association between MTHFR C677T polymorphism and male infertility in Han population of He Nan, China. China Health Care Nutr. 2013;7:629-630.
- Karimian M, Colagar AH. Association of C677T transition of the human methylenetetrahydrofolate reductase (MTHFR) gene with male infertility. Reprod Fertil Dev. 2016;28(6):785-794. Available from: https://doi.org/10.1071/RD14186
- Ni W, Li H, Wu A, Zhang P, Yang H, Yang X, et al. Lack of association between genetic polymorphisms in three folate-related enzyme genes and male infertility in the Chinese population. J Assist Reprod Genet. 2015;32(3):369-374. Available from: https://link.springer.com/article/10.1007/s10815-014-0423-9
- Rai V, Kumar P. Methylenetetrahydrofolate reductase C677T polymorphism and risk of male infertility in Asian population. Ind J Clin Biochem. 2017;32:253-260. Available from: https://doi.org/10.1007/s12291-017-0640-y
- Singh K, Jaiswal D. One-carbon metabolism, spermatogenesis, and male infertility. Reprod Sci. 2013;20:622-630. Available from: https://doi.org/10.1177/1933719112459232
- Cui K, Luan Y, Tang Z, Li CC, Wang T, Wang SG, et al. Human tissue kallikrein-1 protects against the development of erectile dysfunction in a rat model of hyperhomocysteinemia. Asian J Androl. 2019;21:508-515. Available from: https://doi.org/10.4103/aja.aja_111_18
- Salvio G, Ciarloni A, Cutini M, Balercia G. Hyperhomocysteinemia: Focus on endothelial damage as a cause of erectile dysfunction. Int J Mol Sci. 2021;22:418. Available from: https://doi.org/10.3390/ijms22010418
- De Leonardis F, Colalillo G, Finazzi Agrò E, Fuschi A, Asimakopoulos AD. Endothelial dysfunction, erectile deficit, and cardiovascular disease: An overview of the pathogenetic links. Biomedicines. 2022;10:1848. Available from: https://doi.org/10.3390/biomedicines10081848
- Benedetti S, Tagliamonte MC, Catalani S, Benedetti S, Tagliamonte MC, Catalani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online. 2012;25:300-306. Available from: https://doi.org/10.1016/j.rbmo.2012.05.011
- Atig F, Raffa M, Ali HB, Abdelhamid K, Saad A, Ajina M. Altered antioxidant status and increased lipid per-oxidation in seminal plasma of Tunisian infertile men. Int J Biol Sci. 2012;8:139-149. Available from: https://doi.org/10.7150/ijbs.8.139
- Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods, and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371-389. Available from: https://doi.org/10.1093/humupd/dmx006
- Hosen MB, Islam MR, Begum F, Kabir Y, Howlader MZ. Oxidative stress-induced sperm DNA damage, a possible reason for male infertility. Iran J Reprod Med. 2015;13:525-532. Available from: https://pubmed.ncbi.nlm.nih.gov/26568756/
- Aitken RJ, Drevet JR. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants. 2020;9:111. Available from: https://doi.org/10.3390/antiox9020111
- Rashki Ghaleno L, Alizadeh A, Drevet JR, Shahverdi A, Valojerdi MR. Oxidation of sperm DNA and male infertility. Antioxidants. 2021;10:97. Available from: https://doi.org/10.3390/antiox10010097
- Kaltsas A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina (Kaunas). 2023;59:1769. Available from: https://doi.org/10.3390/medicina59101769
- Jurewicz J, Radwan M, Wielgomas B, Sobala W, Piskunowicz M, Radwan P, Bochenek M, Hanke W. The effect of environmental exposure to pyrethroids and DNA damage in human sperm. Systems Biology in Reproductive Medicine. 2015;61(1):37-43. Available from: https://doi.org/10.3109/19396368.2014.981886
- Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur J Obstet Gynecol Reprod Biol. 2019;8(3):100060. Available from: https://doi.org/10.1016/j.eurox.2019.100060
- Cooper HE, Spellacy WN, Prem KA, Cohen WD. Hereditary factors in the Stein-Leventhal syndrome. Am J Obstet Gynecol. 1968;100(3):371-387. Available from: https://doi.org/10.1016/s0002-9378(15)33704-2
- Glueck CJ, Wang P, Fontaine RN, Sieve-Smith L, Tracy T, Moore SK. Plasminogen activator inhibitor activity: An independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metabolism. 1999;48(12):1589-1595. Available from: https://doi.org/10.1016/s0026-0495(99)90250-0
- Jiao X, Chen W, Zhang J, Wang W, Song J, Chen D, et al. Variant alleles of the ESR1, PPARG, HMGA2, and MTHFR genes are associated with polycystic ovary syndrome risk in a Chinese population: A case-control study. Front Endocrinol. 2018;9:504. Available from: https://doi.org/10.3389/fendo.2018.00504
- Almukhtar AA, Almohaidi AMS. Investigation for variation in MTHFR gene in Iraqi Arab females with PCOS. Asian J Microbiol Biotechnol Environ Sci. 2019;21(4):851-861.
- Rai V, Kumar P. Association between methylenetetrahydrofolate reductase gene C677T polymorphism and susceptibility to polycystic ovary syndrome. Ind J Clin Biochem. Available from: https://doi.org/10.1007/s12291-024-01200-4
- Rai V. Methylenetetrahydrofolate reductase C677T polymorphism and recurrent pregnancy loss risk in Asian population: A meta-analysis. Ind J Clin Biochem. 2016;31(4):402-413. Available from: https://doi.org/10.1007/s12291-016-0554-0
- Ahangari N, Doosti M, Mousavifar N, Attaran M, Shahrokhzadeh S, Memarpour S, et al. Hereditary thrombophilia genetic variants in recurrent pregnancy loss. Arch Gynecol Obstet. 2019;300(3):777-782. Available from: https://doi.org/10.1007/s00404-019-05224-7
- Trifonova EA, Swarovskaya MG, Ganzha OA, Voronkova OV, Gabidulina TV, Stepanov VA. The interaction effect of angiogenesis and endothelial dysfunction-related gene variants increases the susceptibility of recurrent pregnancy loss. J Assist Reprod Genet. 2019;36:717-726. Available from: https://doi.org/10.1007/s10815-019-01403-2
- Zarfeshan FY, Kooshkaki O, Kordi TD, Anani SG. Investigation of the association between C677T polymorphism of the MTHFR gene and plasma homocysteine level in recurrent fetal miscarriage. J Obstet Gynaecol Res. 2019;45:1442-1447. Available from: https://doi.org/10.1111/jog.13989
- Shaker MM, Shalabi TA, Amr KS. Correlation of methylation status in MTHFR promoter region with recurrent pregnancy loss. J Genet Eng Biotechnol. 2021;19:44. Available from: https://doi.org/10.1186/s43141-021-00147-w
- Biswas A, Choudhry P, Mittal A, Meena A, Ranjan R, Choudhry VP, et al. Recurrent abortions in Asian Indians: No role of factor V Leiden Hong Kong/Cambridge mutation and MTHFR polymorphism. Clin Appl Thromb Hemost. 2008;14:102-104. Available from: https://doi.org/10.1177/1076029607303774
- Mishra J, Talwar S, Kaur L, Chandiok K, Yadav S, Puri M, et al. Differential global and MTHFR gene specific methylation patterns in preeclampsia and recurrent miscarriages: A case-control study from North India. Gene. 2019;704:68-73. Available from: https://doi.org/10.1016/j.gene.2019.04.036
- Joksic I, Mikovic Z, Filimonovic D, Munjas J, Karadzov ON, Egic A, et al. Combined presence of coagulation factor XIII V34L and plasminogen activator inhibitor 1 4G/5G gene polymorphisms significantly contribute to recurrent pregnancy loss in Serbian population. J Med Biochem. 2020;39:199-207. Available from: https://doi.org/10.2478/jomb-2019-0028
- Wen Y, He H, Zhao K. Thrombophilic gene polymorphisms and recurrent pregnancy loss: A systematic review and meta-analysis. J Assist Reprod Genet. 2023;40:1533-1558. Available from: https://doi.org/10.1007/s10815-023-02823-x
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Preeclampsia. Lancet. 2010;376:631-644. Available from: https://doi.org/10.1016/s0140-6736(10)60279-6
- Redman CW, Sargent IL. Placental stress and preeclampsia: A revised view. Placenta. 2009;30(Suppl A):S38-S42. Available from: https://doi.org/10.1016/j.placenta.2008.11.021
- Chedraui P, Andrade ME, Salazar-Pousada D, Escobar GS, Hidalgo L, Ramirez C, et al. Polymorphisms of the methylenetetrahydrofolate reductase gene (C677T and A1298C) in the placenta of pregnancies complicated with preeclampsia. Gynecol Endocrinol. 2015;31(7):569-572. Available from: https://doi.org/10.3109/09513590.2015.1031104
- Zhou L, Cheng L, He Y, Gu Y, Wang Y, Wang C. Association of gene polymorphisms of FV, FII, MTHFR, SERPINE1, CTLA4, IL10, and TNFalpha with pre-eclampsia in Chinese women. Inflamm Res. 2016;65(9):717-724. Available from: https://doi.org/10.1007/s00011-016-0953-y
- Jafari A, Parchami S, Reiisi S, Miraj S. Association of plasma homocysteine, folic acid levels, and C677T polymorphism in methylene tetra hydrofolate reductase with risk of preeclampsia: A case-control study in Iranian women. Clin Exp Obstet Gynecol. 2018;45(3):367-374. Available from: https://doi.org/10.12891/ceog4032.2018
- Zhang J, Han L, Li W, Chen Q, Lei J, Long M, Yang W, Li W, Zeng L, Zeng S. Early prediction of preeclampsia and small for-gestational-age via multi-marker model in Chinese pregnancies: A prospective screening study. BMC Pregnancy Childbirth. 2019;19:304. Available from: https://doi.org/10.1186/s12884-019-2455-8
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161. Available from: https://doi.org/10.1016/s0140-6736(12)60560-1
- Monangi NK, Brockway HM, House M, Zhang G, Muglia L. The genetics of preterm birth: Progress and promise. Semin Perinatol. 2015;39:574-583. Available from: https://doi.org/10.1053/j.semperi.2015.09.005
- Stalman SE, Solanky N, Ishida M, Alemán-Charlet C, Abu-Amero S, Alders M, Alvizi L, Baird W, Demetriou C, Henneman P, et al. Genetic analyses in small-for-gestational-age newborns. J Clin Endocrinol Metab. 2018;103:917-925. Available from: https://doi.org/10.1210/jc.2017-01843
- Anil KC, Basel PL, Singh S. Low birth weight and its associated risk factors: Health facility-based case-control study. PLoS ONE. 2020;15:e0234907. Available from: https://doi.org/10.1371/journal.pone.0234907
- Dahman HAB. Risk factors associated with preterm birth: A retrospective study in Mukalla Maternity and Childhood Hospital, Hadhramout Coast, Yemen. Sudan J Paediatr. 2020;20:99-110. Available from: https://doi.org/10.24911/sjp.106-1575722503
- Sukla KK, Tiwari PK, Kumar A, Raman R. Low birthweight (LBW) and neonatal hyperbilirubinemia (NNH) in an Indian cohort: Association of homocysteine, its metabolic pathway genes and micronutrients as risk factors. PLoS ONE. 2013;8:e71587. Available from: https://doi.org/10.1371/journal.pone.0071587
- Wang BJ, Liu MJ, Wang Y, Dai JR, Tao JY, Wang SN, Zhong N, Chen Y. Association between SNPs in genes involved in folate metabolism and preterm birth risk. Genet Mol Res. 2015;14(1):850-859. Available from: https://doi.org/10.4238/2015.february.2.9
- Hwang IW, Kang YD, Kwon BN, Hong JH, Han SH, Kim JS, Park JW, Jin HJ. Genetic variations of MTHFR gene and their association with preterm birth in Korean women. Medicina. 2017;53:380-385. Available from: https://doi.org/10.1016/j.medici.2018.01.001
- Tiwari D, Bose PD, Das S, Das CR, Datta R, Bose S. MTHFR (C677T) polymorphism and PR (PROGINS) mutation as genetic factors for preterm delivery, fetal death and low birth weight: A Northeast Indian population-based study. Meta Gene. 2015;3:31-42. Available from: https://doi.org/10.1016/j.mgene.2014.12.002
- Kramer MS, Goulet L, Lydon J, Séguin L, McNamara H, Dassa C, et al. Socio-economic disparities in preterm birth: Causal pathways and mechanisms. Paediatr Perinat Epidemiol. 2001;15(Suppl. 2):104-123. Available from: https://doi.org/10.1046/j.1365-3016.2001.00012.x
- Nan Y, Li H. MTHFR genetic polymorphism increases the risk of preterm delivery. Int J Clin Exp Pathol. 2015;8:7397-7402. Available from: https://pubmed.ncbi.nlm.nih.gov/26261642/
- Wang BJ, Liu MJ, Wang Y, Dai JR, Tao JY, Wang SN, Zhong N, Chen Y. Association between SNPs in genes involved in folate metabolism and preterm birth risk. Genet Mol Res. 2015;14(1):850-859. Available from: https://doi.org/10.4238/2015.february.2.9
- van der Put NM, Eskes TK, Blom HJ. Is the common 677CT mutation in the methylenetetrahydrofolate reductase gene a risk factor for neural tube defects? A meta-analysis. Q J Med. 1997;90:111-115. Available from: https://doi.org/10.1093/qjmed/90.2.111
- Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137-146. Available from: https://doi.org/10.1016/s0166-2236(03)00032-8