More Information
Submitted: October 05, 2024 | Approved: October 21, 2024 | Published: October 22, 2024
How to cite this article: Ghafarizade AA, Shojafar E, Naderi S, Seifi F, Noshad A, Lavasani Z, et al. The Clinical Pregnancy and Live Birth Following Transfer of One Arrested Embryo: A Case Report. Clin J Obstet Gynecol. 2024; 7(4): 112-114. Available from: https://dx.doi.org/10.29328/journal.cjog.1001175
DOI: 10.29328/journal.cjog.1001175
Copyright License: © 2024 Ghafarizade AA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: ICSI; Embryo transfer; Pregnancy; Live birth
The Clinical Pregnancy and Live Birth Following Transfer of One Arrested Embryo: A Case Report
Ali Asghar Ghafarizade1, Elham Shojafar1*, Samira Naderi1, Fatemeh Seifi1, Alireza Noshad1, Zohreh Lavasani1, Zahra Kalhori2 and Elahe Ghadiri1
1Rastak Infertility Treatment Center, Sina Hospital, Arak, Iran
2Department of Biology, Faculty of Science, Razi University, Kermanshah, Iran
*Address for Correspondence: Elham Shojafar, Rastak Infertility Treatment Center, Sina Hospital, Arak, Iran, Email: [email protected]
Background: One of the problems in vitro fertilization (IVF) treatment for infertility is the high frequency of embryo developmental arrest in the preimplantation stages. Arrested embryos were not selected for transfer and were usually discarded.
Case report: We present a case of clinical pregnancy and live birth following IVF treatment and transfer of one arrested embryo. A 31-year-old woman with unexplained infertility underwent IVF treatment. Using the IVF procedure, 7 embryos were produced which were frozen on day 3. In order to embryo transfer in the blastocyst stage, two embryos were thawed and cultured for 2 days. After thawing, one of them was not suitable for transfer and another embryo was arrested at the 10-12 cell stage.
Discussion: The Clinical pregnancy and live birth happened after the transfer of an arrested embryo on day 5.
Conclusion: This case showed that arrested embryos may resume growth after the transfer to the uterus and result in a successful pregnancy and live birth.
In humans, less than 50% of in-vitro-produced embryos grow to the blastocyst stage [1-3], and 50% of in vitro-produced embryos are arrested during the early cleavages in the first week of development [4,5]. In infertile couples who are undergoing IVF treatment, the production of arrested embryos is very common. About 40% of infertile couples receiving IVF treatment have at least one arrested embryo [1]. The incidence of embryonic arrest can be due to several reasons. The high metabolic activity in the embryo, which can lead to Reactive oxygen species (ROS) production, is one of the reasons for embryo arrest [1,6]. Chromosomal abnormalities and single gene disorders are other causes of embryo developmental arrest [7]. About 68% of arrested embryos are aneuploidy. In addition, the higher rates of mosaicism are seen in these embryos [8]. It seems incidence of embryonic developmental arrest may be the mechanism to prevent the development of some chromosomally abnormal embryos [1]. Arrested embryos are not considered suitable embryos for transfer and are usually discarded [8-10]. However, in arrested embryos, activity of metabolism and absence of apoptosis [1,11,12], have been shown and these embryos have also been used as a source of human stem cells [13]. Derived human embryonic stem cells from arrested embryos have the ability to differentiate into derivates of all three embryonic germ layers [13]. Also, sub-culture environments can induce embryo developmental arrest [14-16], and changing these environmental conditions may lead to embryo growth resumption. So it may be said that some of the arrested embryos can be a chance of having a baby in infertile couples.
A 31-year-old woman and her husband with unexplained infertility and unremarkable medical history underwent ICSI treatment in our Fertility Clinic in 2023.
Using two vials of 150 IU recombinant FSH plus 75 IU recombinant LH (Pergoveris, USA) the ovarian stimulation was started at day 2 of the cycle. Cetrorelix acetate as GnRH antagonist (Cetrotide) was injected when the largest follicle was ≥14mm. All the medications were continued until at least one follicle reached ≥18 mm in diameter, and then 2 doses of triptorelin (Decapeptyl, Spain) were administered. The puncture of the ovaries was done and 10 oocytes were retrieved. 7 of 10 oocytes were mature (metaphase II; MII) and suitable for ICSI. After ICSI the embryos were cultured in 30 µl droplets of SAGE 1-Step medium (Origio, Denmark) for 72h. After 3 days, 7 embryos developed and were cryopreserved. For embryo transfer, after the first menstrual cycle, using estradiol valerait (Abureihan, Iran) at a dose of 6mg/day for 1 week, the endometrium preparation was down, and when the endometrium thickness was ≥7, micronized progesterone (Abureihan, Iran) at a dose of 900 mg/day for 5 days was vaginally added. Selecting the best embryo for transfer, embryos were grown to the blastocyst stage. So two days before embryo transfer, 2 embryos were thawed and cultured in 30µl droplets of SAGE 1-Step medium for 48h.
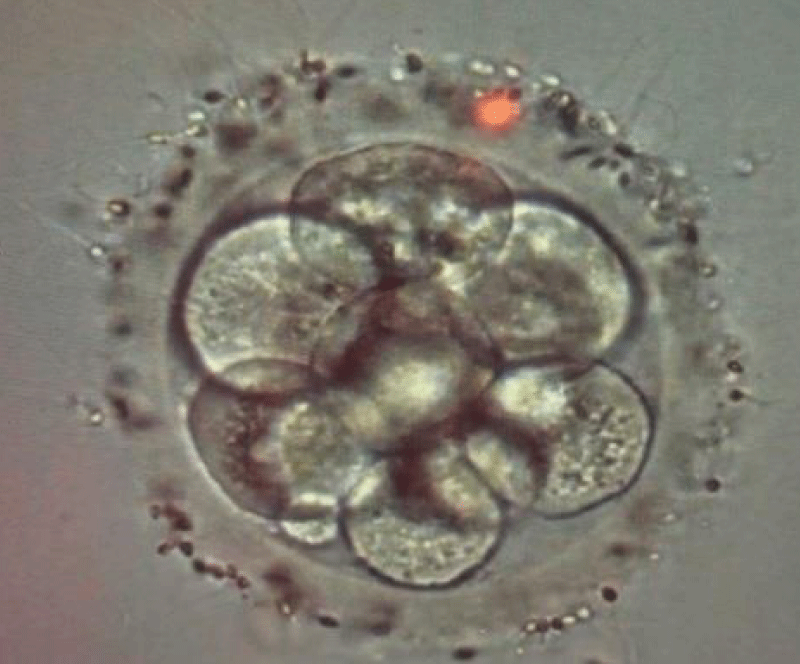
2 days after thawing one embryo was arrested in the 10-12 cell stage and another embryo was not suitable for transfer (Figure 1). Since no blastomere from the 10-12cell stage embryo had divided during the last 24-26 hours, this embryo was considered an arrested embryo [4,13,17]. According to the patient's decision the arrested embryo was transferred, which led to pregnancy and a healthy female baby delivered at 37 weeks’ gestation.
Figure 1: The arrested embryo on day 5 before transfer (×400).
The arrested embryos are not considered suitable for transfer and are discarded [8-10]. Since embryonic developmental arrest in preimplantation stages is common in in vitro culture conditions, embryo arrest is one of the factors that reduces the chance of infertile couples having children. The most common reasons for embryo developmental arrest are aneuploidy and single-gene mutations [7,8]. However, studies have shown that oxidative stress and suboptimal environmental conditions can also induce embryo developmental arrest [1,6,15]. Free radical oxygen production can induce embryo developmental arrest by promoting blastomere senescence [18,19] and shortening the telomere [20,21]. Therefore, some arrested embryos, are euploid and may resume growth as the culture conditions are improved and oxidative stress relieved [1]. In this case, the change in embryo culture condition after embryo transfer was probably the cause of growth resumption.
A study by Racowskym, et al. on the time of embryo transfer showed no pregnancy following the transfer of arrested embryos [22]. In 2003, Virant-Klunm, et al. Reported 2% of pregnancies after the transfer of arrested embryos, which eventually led to miscarriage [23]. This is the first report of the transfer of an arrested embryo leading to the birth of a healthy baby.
Limitation
The limitation of our study is the low number of cases analyzed.
As far as we know, this is the first study to show a case of clinical pregnancy and live birth following IVF treatment and transfer of one arrested embryo. This case showed that arrested embryos may resume growth after the transfer to the uterus and result in a successful pregnancy and live birth. It seems that by changing the culture conditions, some of the euploid-arrested embryos may resume growth and can be selected for transfer. We conclude that it would be useful to perform arrested embryos during IVF treatment, especially for patients with a low number of embryos. However, further studies on this subject should focus on the precise mechanism of the protecting properties of arrested embryos leading to the birth of a healthy baby.
Ethical consideration
The authors declare that informed patient consent was provided.
- Sfakianoudis K, Maziotis E, Karantzali E, Kokkini G, Grigoriadis S, Pantou A, et al. Molecular drivers of developmental arrest in the human preimplantation embryo: a systematic review and critical analysis leading to mapping future research. Int J Mol Sci. 2021;22(15):8353. Available from: https://doi.org/10.3390/ijms22158353.
- Xu KP, Yadav BR, Rorie RW, Plante L, Betteridge KJ, King WA. Development and viability of bovine embryos derived from oocytes matured and fertilized in vitro and co-cultured with bovine oviducal epithelial cells. J Reprod Fertil. 1992;94(1):33-43. Available from: https://doi.org/10.1530/jrf.0.0940033.
- McCollin A, Swann RL, Summers MC, Handyside AH, Ottolini CS. Abnormal cleavage and developmental arrest of human preimplantation embryos in vitro. Eur J Med Genet. 2020;63(2):103651. Available from: https://doi.org/10.1016/j.ejmg.2019.04.008.
- Hardy K, Spanos S, Becker D, Iannelli P, Winston RM, Stark J. From cell death to embryo arrest: mathematical models of human preimplantation embryo development. Proc Natl Acad Sci U S A. 2001;98(4):1655–1660. Available from: https://doi.org/10.1073/pnas.98.4.1655.
- Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M, et al. Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod Biomed Online. 2017;34(2):137-146. Available from: https://doi.org/10.1016/j.rbmo.2016.11.008.
- Favetta LA, St John EJ, King WA, Betts DH. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic Biol Med. 2007;42(8):1201–1210. Available from: https://doi.org/10.1016/j.freeradbiomed.2007.01.018.
- Solovova OA, Chernykh VB. Genetics of oocyte maturation defects and early embryo development arrest. Genes. 2022;13(11):1920. Available from: https://doi.org/10.3390/genes13111920.
- McCoy RC, Summers MC, McCollin A, Ottolini CS, Ahuja K, Handyside AH. Meiotic and mitotic aneuploidies drive arrest of in vitro fertilized human preimplantation embryos. Genome Med. 2023;15(1):77. Available from: https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-023-01231-1.
- Liu L, Cai J, Li P, Chen Y, Sha A, Ren J. Clinical outcome of IVF/ICSI cycles with an arrested embryo on day 3. Int J Clin Exp Med. 2016;9(8):16414-16424. Available from: https://e-century.us/files/ijcem/9/8/ijcem0025986.pdf.
- Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in vitro. Hum Reprod. 2000;15(12):2634-2643. doi: 10.1093/humrep/15.12.2634. Available from: https://doi.org/10.1093/humrep/15.12.2634.
- Matwee C, Betts DH, King WA. Apoptosis in the early bovine embryo. Zygote. 2000;8(1):57–68. doi: 10.1017/s0967199400000836. Available from: https://doi.org/10.1017/s0967199400000836.
- Gjorret JO, Knijn HM, Dieleman SJ, Avery B, Larsson LI, Maddox-Hyttel P. Chronology of apoptosis in bovine embryos produced in vivo and in vitro. Biol Reprod. 2003;69(4):1193–1200. doi: 10.1095/biolreprod.102.013243. Available from: https://doi.org/10.1095/biolreprod.102.013243
- Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24(12):2669-2676. doi: 10.1634/stemcells.2006-0377. Available from: https://doi.org/10.1634/stemcells.2006-0377.
- Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16(1):31–38. Available from: https://doi.org/10.1002/bies.950160105.
- Betts DH, King WA. Genetic regulation of embryo death and senescence. Theriogenology. 2001;55(1):171–191Available from: https://doi.org/10.1016/s0093-691x(00)00453-2.
- Favetta LA, Robert C, King WA, Betts DH. Expression profiles of p53 and p66shc during oxidative stress-induced senescence in fetal bovine fibroblasts. Exp Cell Res. 2004;299(1):36–48. Available from: https://doi.org/10.1016/j.yexcr.2004.05.009.
- Munné S, Grifo J, Cohen J, Weier HU. Chromosome abnormalities in human arrested preimplantation embryos: a multiple-probe FISH study. Am J Hum Genet. 1994;55(1):150-159. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC1918237/.
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35(8):927–945. Available from: https://doi.org/10.1016/s0531-5565(00)00180-7.
- von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000;908:99–110. Available from: https://doi.org/10.1111/j.1749-6632.2000.tb06639.x.
- Li GZ, Eller MS, Firoozabadi R, Gilchrest BA. Evidence that exposure of the telomere 3' overhang sequence induces senescence. Proc Natl Acad Sci U S A. 2003;100(2):527–531. Available from: https://doi.org/10.1073/pnas.0235444100.
- Stewart SA, Ben-Porath I, Carey VJ, O’Connor BF, Hahn WC, Weinberg RA. Erosion of the telomeric single-strand overhang at replicative senescence. Nat Genet. 2003;33(4):492–496. Available from: https://doi.org/10.1038/ng1127.
- Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsburg ES. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73(3):558-564. Available from: https://doi.org/10.1016/s0015-0282(99)00565-8.
- Virant-Klun I, Tomazevic T, Zorn B, Bacer-Kermavner L, Mivsek J, Meden-Vrtovec H. Blastocyst formation—good indicator of clinical results after ICSI with testicular spermatozoa. Hum Reprod. 2003;18(5):1070-1076. Available from: https://doi.org/10.1093/humrep/deg