More Information
Submitted: August 07, 2024 | Approved: August 28, 2024 | Published: August 29, 2024
How to cite this article: Ajao FO, Yusuf OO, Balogun DA, Iyedupe MO, Adesola MO, Egunjobi GA. Linagliptin Efficacy on Hyperglycemia, Oxidative Stress, and Inflammation in Gestational Diabetes Mellitus. Clin J Obstet Gynecol. 2024; 7(3): 093-099. Available from: https://dx.doi.org/10.29328/journal.cjog.1001171
DOI: 10.29328/journal.cjog.1001171
Copyright License: © 2024 Ajao FO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Linagliptin; Gestational diabetes mellitus; Hyperglycemia; Oxidative stress; Inflammation
Linagliptin Efficacy on Hyperglycemia, Oxidative Stress, and Inflammation in Gestational Diabetes Mellitus
Folasade Omobolanle Ajao*, Oluwatobi Olayiwola Yusuf, Damilola Ayodeji Balogun, Marcus Olaoye Iyedupe, Mariam Olayinka Adesola and George Adetomiwa Egunjobi
Physiology Department, Faculty of Basic Medical Science, College of Health Science, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria
*Address for Correspondence: Folasade Omobolanle Ajao, Department of Physiology, Ladoke Akintola University of Technology, P.M.B. 4000 Ogbomoso, 210214, Nigeria, Email: [email protected]
Background: Linagliptin is an anti-diabetic drug that claims no adverse effects and treatment of gestational diabetes mellitus (GDM) demands a safe anti-diabetic medication. Therefore, this study investigates the anti-diabetic efficacy of linagliptin in an induced GDM.
Materials and methods: Thirty-two matured female rats (100 - 200 g) were utilized. Sixteen non-pregnant/diabetic animals were fed with a normal diet and sixteen rats were fed with a high-fat (HFD), mated at the estrous stage in 2:1, and pregnancy was confirmed with a spermatozoa in a vaginal smear. The pregnant rats were intraperitoneally injected with a single dose (30 mg/kgb. wt) of streptozotocin (STZ) to induce GDM. The animals were grouped into 4 groups, 8 rats/groups. Group I: control; Group II: control + 10 mg/kgb.wt linagliptin; Group III: GDM; Group IV: GDM + 10 mg/kgb.wt linagliptin. The animals were sacrificed after 14 days of treatment. Blood samples were collected for biochemical parameters.
Results: Fasting blood glucose (FBG) insulin, glycated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), malondialdehyde (MDA), interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-alpha (TNF-α) levels significant (p < 0.05) elevated in GDM rats, with significant reduction in high-density lipoprotein-cholesterol (HDL-C), catalase (CAT), superoxide dismutase (SOD) and reduced glutathione (GSH). Linagliptin administration significantly (p < 0.05) decreased the FBG, insulin, HbA1c, TC, TG, LDL-C, MDA, IL-6, IL-1β, and TNF-α and ameliorates the HDL-C, CAT, SOD, and GSH levels significantly.
Conclusion: Linagliptin remarkably showed anti-hyperglycemic, anti-oxidative, and anti-inflammatory properties. Linagliptin could be a promising drug for hyperglycemia treatment during gestation.
Globally, it is estimated that around 537 million people are currently living with diabetes, with projections expected to increase to more than 643 million people by 2030 [1].
Gestational diabetes mellitus (GDM) is an independent type of diabetes defined as glucose intolerance with first recognition during 2nd and 3rd trimesters of pregnancy and resolves after birth [2]. GDM is one of the most common medical complications of pregnancy which can lead to serious adverse health effects for the mother and child including increased cesarean delivery, type 2 diabetes (T2DM) and cardiovascular disease (CVD) in the mother and future obesity, preterm birth, CVD, T2DM, and GDM in the child [3]. The increase in the progression of GDM has been associated with maternal age, obesity, family history of T2DM, and polycystic ovary syndrome (PCOS) [4].
Oxidative stress induced by chronic hyperglycemia triggers many cascade events resulting in tissue injury, pathological conditions, and the development of GDM [5,6]. The oxidative stress-induced inflammatory response causes the release of inflammatory cytokines that block insulin to regulate glucose metabolism and lead to insulin resistance and GDM manifestation [7,8].
Currently, GDM treatment includes insulin therapy and lifestyle interventions, such as diet and exercise but insulin resistance often accompanies insulin therapy and widely known promising anti-diabetic drugs, such as metformin and glyburide still face the drawback of long-term safety for the mother and child [9].
Linagliptin is a xanthine-based, highly potent, and long-acting non-peptidomimetic DPP-4 inhibitor that is well tolerated in T2DM patients [10]. Linagliptin has been shown to attenuate fat accumulation, inhibit cytokine infiltration, and prevent hepatic steatosis progression in addition to insulin sensitivity and glycemic index enhancement [11]. Linagliptin has demonstrated hypoglycemic properties and non-adverse effects on the cardiac of diabetic patients [12]. Also, research has shown that linagliptin monotherapy for diabetes displayed no negative effects and claimed to exhibit mild to moderate side effects such as diarrhea, coughing, nasopharyngitis, urinary tract infection, and hyperlipidemia when administered with other anti-diabetic medications [13]. However, the mono-pharmacological efficacy of linagliptin on abnormal hyperglycemia during gestation has not been proven. Therefore, this research investigates the linagliptin therapeutic properties in streptozotocin (STZ)-induced GDM rats’ model.
Drugs and chemicals
Glucose, citrate buffer, phosphate-buffered saline, normal saline, streptozotocin, linagliptin, distilled water.
Experimental animals
Thirty-two adult female Wistar rats weighing 100 - 200g were utilized. The animals were kept in a clean polypropylene cage for two weeks to acclimatize with access to standard feed and water ad libitum under a pathogen-free hygienic environment (25 ± 2ºC ), relative humidity (50 ± 5%) and 12:12 hours light/dark cycles. All experimental procedures followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals protocol and approved by the Faculty of Basic Medical Science Ethics Research Committee of Ladoke Akintola University of Technology (Ethical Approval Number: ERCFBMSLAUTECH/062/08/2024)
High-fat diet feed formulation
Composition of High Fat Diet according to Akinlade, et al. [14](Table 1).
Table 1: Composition of High Fat Diet according to Akinlade, et al. [14]. |
|||
| Dietary components | Control diet | High-fat diet | |
| Energy (Kcal/g) | 3.00 | 6.4 | |
| Calorie percentage |
|
|
|
| Carbohydrate | 60 | 30 | |
| Fat | 15 | 65 | |
| Protein | 25 | 5 | |
| Weight percentage |
|
|
|
| Carbohydrate Fat |
15 25 |
40 45 |
|
| Protein | 60 | 15 | |
| Materials | Standard chow diet |
maize, wheat offal, groundnut cake, soya meal, palm kernel cake/ oil, bone meal, methionine, lysine, salt, finisher premix, coupled | |
Animal mating and induction of GDM
After acclimatization, sixteen rats were fed a high-fat diet (HFD) for 8 weeks. The blood glucose levels were checked after being fed with HFD and rats with fasting blood glucose levels >120 mg/dL were excluded before determining the estrous stage. The animals were subjected to overnight fasting and the estrous stage was determined with a vaginal smear. Animals at the estrous stage were mated overnight with a matured active male at a ratio of 2:1 manner. The presence of a copulatory plug was used to confirm mating in the animals and a vaginal smear was done to affirm the presence of spermatozoa using a microscope. The date sperm was observed in the vaginal smear was considered as day 0 of pregnancy.
The pregnant animals were fasted overnight for 12 hours after confirmation of pregnancy and injected with a freshly prepared single dose of streptozotocin (STZ) (30 mg/kgb. wt).
The rats were given a 2% glucose solution to avert drug-induced hypoglycemia death and diabetes induction was confirmed in the pregnant rats after 72 hours of STZ injection using the pricked tail vein blood on a glucometer (Accu-chek). Pregnant rats with fasting blood glucose levels ≥200 mg/dL were selected and used as GDM models in this experiment.
Animal grouping
Sixteen GDM rats and sixteen non-pregnant/diabetic rats were included. The non-pregnant/diabetic rats and GDM rats are grouped into four groups, 8 rats/group as follows:
Group 1: Normal control (non-pregnant/diabetic rats + distilled water)
Group 2: Normal control + 10 mg/kgb. wt linagliptin
Group 3: GDM rats + distilled water
Group 4: GDM rats + 10 mg/kgb. wt linagliptin.
Daily feed and water intake were recorded. Body weight and fasting blood glucose levels were determined weekly. The treatment of the animals lasted for 14 days.
Oral glucose tolerance test
The animals were fasted overnight (14 hours) and were intra-gastrically administered with a glucose solution (2.0 g/kg).
Animals' blood glucose level was checked at consecutive intervals of 0, 30, 60, 90, and 120 m in with a glucometer and recorded.
Biochemical assay
After administering the last linagliptin dose, the animals were allowed to fast overnight (12 hours), then anesthetized with a single dose of ketamine (40 mg/kg) and xylazine (20 mg/kg) injected intraperitoneally and sacrificed by cervical dislocation. Blood samples were collected by cardiac puncture and centrifuged at 3500 rpm for 15 mins at - 4oC. The clear supernatant plasma retrieved was used for the biochemical parameters estimation.
Glucose-oxidase/peroxidase (GOD-POD) method was used to measure the plasma fasting blood glucose level via a drop of pricked-tail blood on a glucometer.
The Homeostasis Model Assessment of insulin resistance (HOMA-IR) was utilized to assess insulin resistance. HOMA-IR = fasting glucose (mmol/l) x fasting insulin (μU/ml) / 22.5 Glycated hemoglobin (HbA1c) was determined using rats' HbA1c assay kit following the manufacturer's instructions.
Insulin, interleukin-6 (IL-6), interleukin-1β (IL-1β) tumor necrosis factor-alpha (TNF-α), malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH) levels were determined using Enzymes-Linked Immunosorbent Assay (ELISA) with individual ELISA kits according to the manufacturer’s protocols.
Enzymatic colorimetric methods were used to estimate the total cholesterol (TC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) with commercially available kits, according to the manufacturer’s guidelines. Low-density lipoprotein cholesterol (LDL-C) was calculated based on the Friedewald et al equation: LDL-C= TC - (HDL-C + TG/5) [15].
Statistical analysis
The data was analyzed using GraphPad Prism (version 10.2.0) and the results were expressed as the mean ± SEM and statistical comparisons among the groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. A p < 0.05 was considered statistically significant.
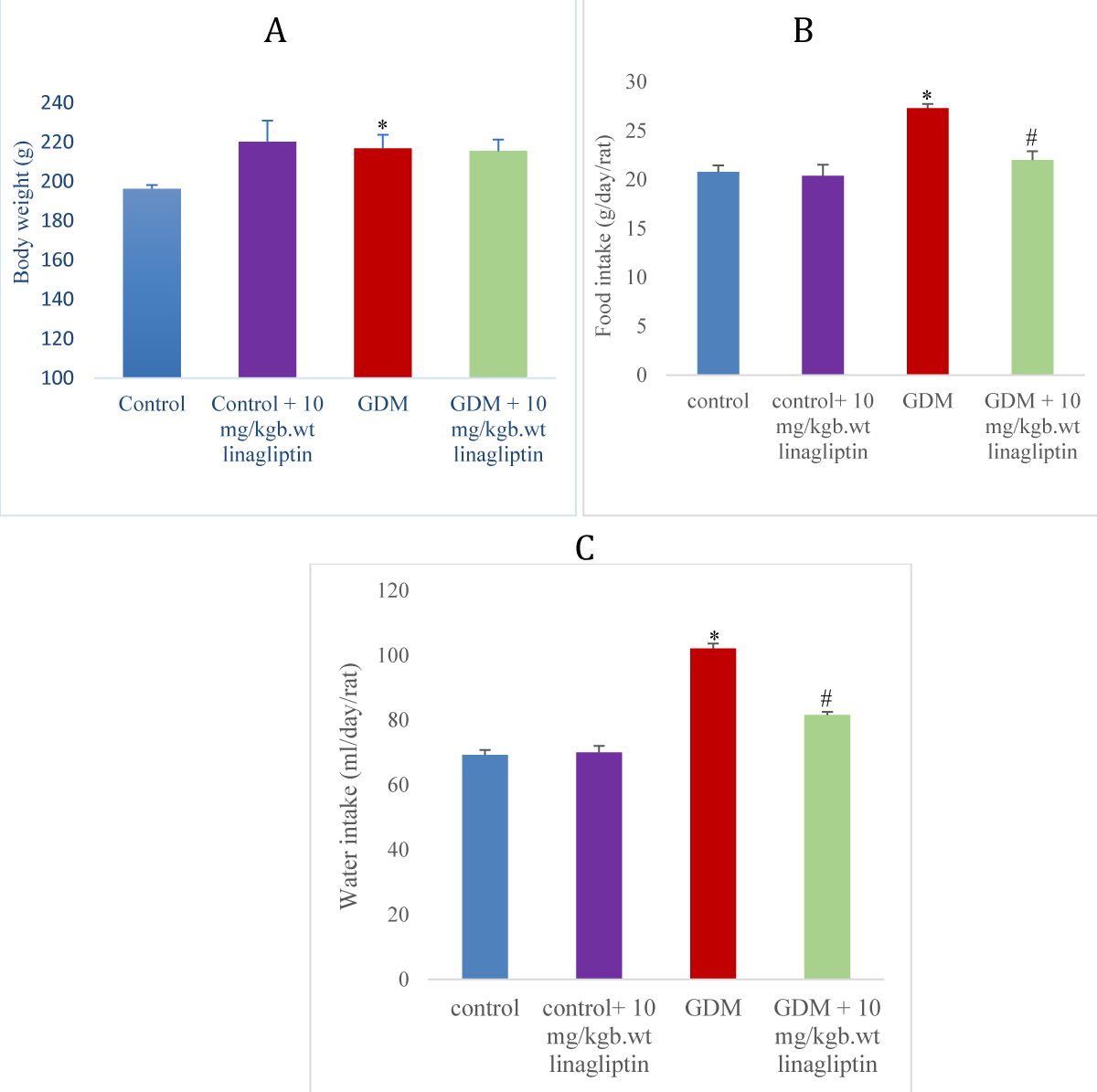
Effect of linagliptin on body weight in HFD/STZ-induced GDM rats
The GDM rats showed a slightly significant (p < 0.05) increase in body weight compared to normal control and a reduction in the body in comparison with the control treated with linagliptin. Oral gavage of GDM rats with 10 mg/kgb.wt had no significant difference in body weight in comparison with the GDM untreated group (Figure 1A).
Effect of linagliptin on food and water intake in HFD/STZ-induced GDM rats
There was a significant increase (p < 0.05) in feed and water intake of the GDM group when compared to the normal control and control treated with linagliptin. Administration of 10 mg/kgb.wt linagliptin to the GDM group significantly decreased the feed and water intake when compared with the GDM untreated group (Figure 1 B, C).
Figure 1: Effect of linagliptin on (A) body weight (B) food intake (C) water intake in HFD/STZ-induced GDM rats. Values are expressed as mean ± SEM (n = 8). *significant at p < 0.05 compared with the control; *significant at p < 0.05 compared with control + linagliptin; #significant at p < 0.05 compared with GDM untreated group.
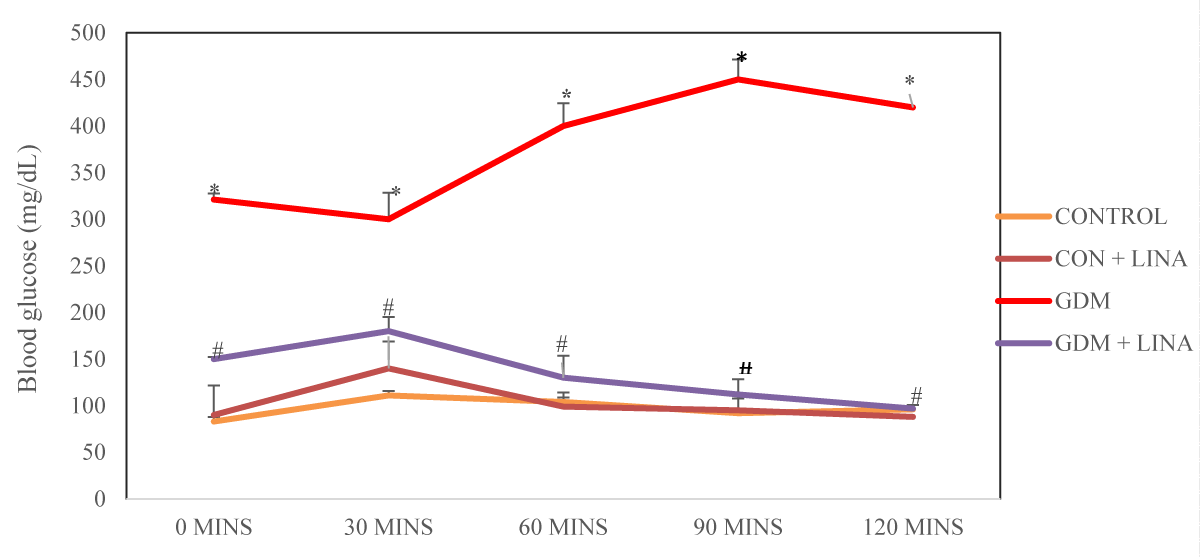
Effect of linagliptin on oral glucose tolerance in HFD/STZ-induced GDM rats
The rats with GDM had a significant (p < 0.05) increase in oral glucose tolerance when compared with control and control administered with linagliptin. Treatment of GDM rats with linagliptin significantly diminished the oral glucose tolerance levels in comparison with untreated GDM group (Figure 2).
Figure 2: Effect of linagliptin on oral glucose tolerance test in HFD/STZ-induced GDM rats. Values are expressed as mean ± SEM (n = 8). *significant at p < 0.001 compared with the control; *significant at p < 0.001compared with control + linagliptin; #significant at p < 0.001 compared with GDM untreated group.
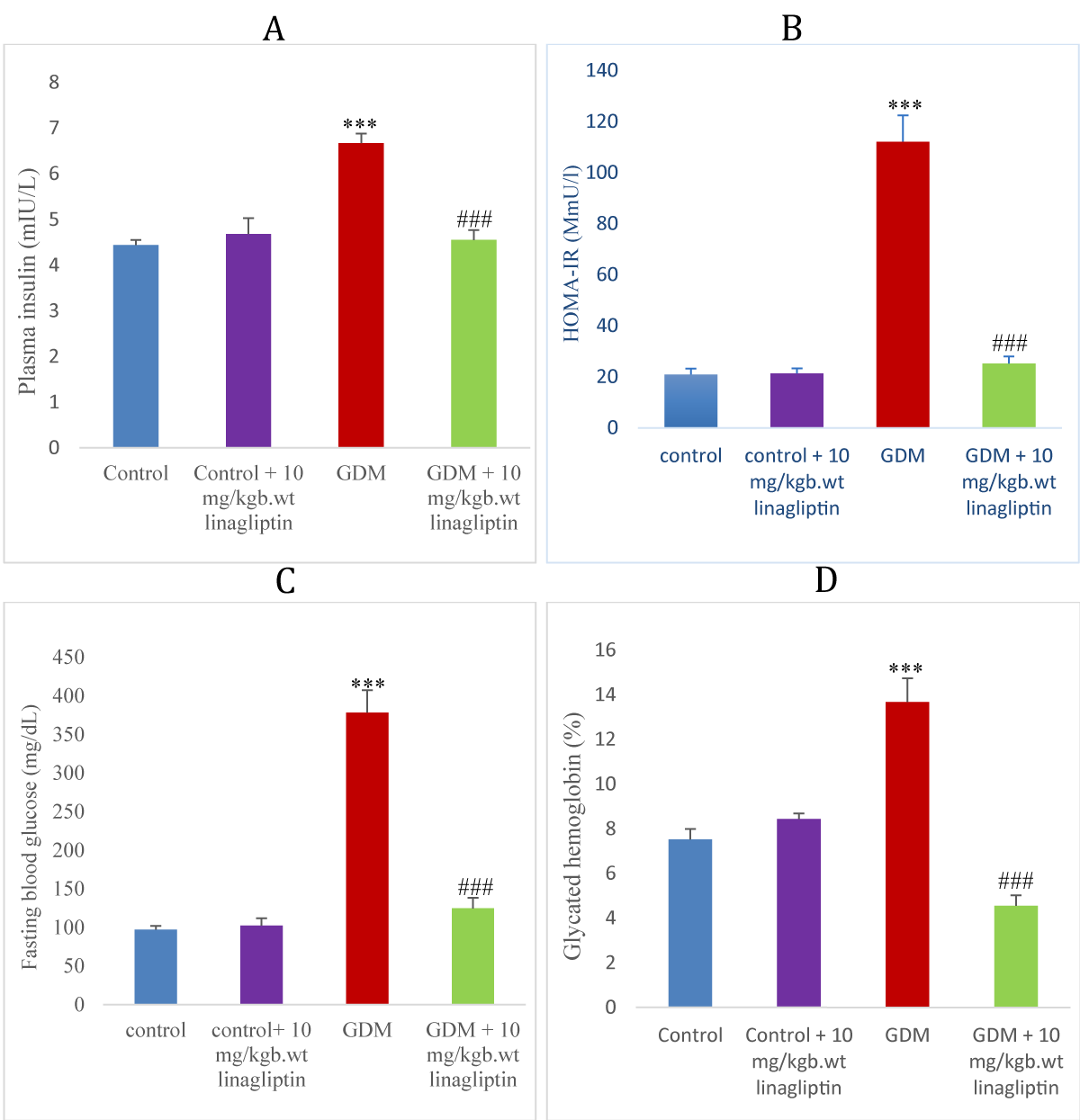
Effect of linagliptin on insulin, HOMA-IR, fasting blood glucose, and glycated hemoglobin in HFD/STZ-induced GDM rats
GDM rats showed a significant increase (p < 0.001) in insulin, HOMA-IR, fasting blood glucose levels, and glycated hemoglobin when compared with normal control and control treated with linagliptin. Treatment of GDM rats with linagliptin 10 mg/kgb.wt significantly decreased the insulin, HOMA-IR,
fasting blood glucose levels, and glycated hemoglobin compared to the GDM untreated group (Figure 3 A-D).
Figure 3: Effect of linagliptin on (A) plasma insulin (B) HOMA-IR (C) fasting blood glucose (D) glycated hemoglobin in HFD/STZ-induced GDM rats. Values are expressed as mean ± SEM (n = 8). ***significant at p < 0.001 compared with the control; ***significant at p < 0.05 compared with control + linagliptin; ###significant at p < 0.001 compared with GDM untreated group.
Effect of linagliptin on the lipid profile in HFD/STZ-induced GDM rats
The levels of TG, TC, and LDL were significantly increased (p < 0.05), and a significant decrease in HDL levels of the GDM group compared to the control and control treated with linagliptin. Treatment of the GDM group with linagliptin significantly decreased the levels of TG, TC, and LDL and increased the HDL level in comparison to the GDM untreated group (Table 2).
| Table 2: Effect of linagliptin on Lipid profile, Antioxidants, and Inflammatory cytokines in HFD/STZ-induced GDM rats. | ||||
| Groups/Parameters | Normal control | Control + 10 mg/kgb.wt linagliptin | GDM control | GDM + 10 mg/kgb.wt linagliptin |
| TC (mmol/l) | 1.74 ± 0.19 | 1.72 ± 0.15 | 3.31 ± 0.39* | 1.62 ± 0.05# |
| TG (mmol/l) | 1.14 ± 0.08 | 1.36 ± 0.02 | 1.95 ± 0.14* | 1.15 ± 0.06# |
| LDL-C (mmol/l) | 0.87 ± 0.07 | 0.73 ± 0.09 | 1.28 ± 0.15* | 0.55 ± 0.09# |
| HDL-C (mmol/l) | 0.82 ± 0.11 | 0.72 ± 0.06 | 0.47 ± 0.03* | 0.84 ± 0.06# |
| MDA (µM) | 1.57 ± 0.26 | 1.63 ± 0.11 | 2.41 ± 0.25* | 1.67 ± 0.19# |
| SOD (U/ml) | 1.29 ± 0.08 | 1.25 ± 0.03 | 0.78 ± 0.19* | 1.25 ± 0.08# |
| CAT (u/mg) | 19.54 ± 2.01 | 18.81 ± 1.44 | 14.26 ± 0.58* | 17.84 ± 1.72# |
| GSH (mM) | 0.15 ± 0.01 | 0.17 ± 0.02 | 0.03 ± 0.00* | 0.18 ± 0.03# |
Effect of linagliptin on oxidative stress biomarker and antioxidants in HFD/STZ-induced GDM rats
The GDM rats displayed a significant (p < 0.05) increase in CAT, GSH, and SOD levels and a significant increase in MDA levels compared to the control and control administered with linagliptin. Treatment of GDM rats with linagliptin significantly increased the CAT, GSH, and SOD levels and decreased the MDA level in comparison to the GDM untreated group (Table 2).
Effect of linagliptin on inflammatory cytokines in HFD/STZ-induced GDM rats
The level of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) significantly (p < 0.05) increased in the GDM group compared to the control and control administered with linagliptin. Treatment of GDM rats with linagliptin significantly lessens the TNF-α, IL-6, and IL-1β levels compared to the GDM untreated group (Table 2).
Gestational diabetes mellitus (GDM) is a common pregnancy complication with spontaneous hyperglycemia during pregnancy [16]. GDM occurs in more than 10% of pregnant women. The prevalence of GDM is increasing worldwide with elevated incidence of obesity and maternal age [17]. GDM had pathophysiology and similar features to T2DM. The mechanism of GDM is not clearly understood due to the complexity of pregnancy. In recent years, increasing studies have focused on the investigation of novel therapeutic strategies for GDM [18]. The present research investigated the anti-hyperglycemic potential of linagliptin in an induced GDM rats’ model.
Hyperglycemia, hyperinsulinemia, glucose intolerance, insulin resistance, and body weight gain are pregnancy-related conditions linked with an elevated risk of parturition complications in gestational diabetes mellitus [19]. In accord with Abdel Aziz, et al. findings [20], elevated blood glucose and reduced body weight gain accompanied by high HOMA-IR were manifested in the GDM of this study. However, linagliptin remarkably reduced blood glucose and attenuated insulin resistance as revealed by HOMA-IR results, this demonstrated that linagliptin facilitates insulin sensitivity in peripheral tissues for efficient glucose uptake, corroborating the findings of Siddiqui, et al. [21]. Physiological change in body mass during gestation might be responsible for the non-significant difference in the body of diabetic rats administered with linagliptin, and non-structural proteolysis may be attributed to the body weight gain in non-gestation diabetic rats.
Glycated hemoglobin is a predictor of diabetes condition [16]. The elevated glycated hemoglobin level seen in GDM predicts a diabetic state. Linagliptin treatment decreased hemoglobin glycation, which could be attributed to the diminishing of circulating peripheral blood glucose and reduction of glucagon production, which stimulates hepatic gluconeogenesis, which accords with the report of Del Prato, et al. [22].
Normal pregnancies are closely associated with temporary alterations in the lipid profile, including increased levels of total cholesterol, LDL, and HDL, and decreased levels of triglycerides [23]. Variations in the lipid profile of GDM women have been reported. Lipid levels and lipid hydroperoxide activities were greater in the third trimester in women with GDM [24]. Abnormally elevated plasma lipids that occur during a typical pregnancy are referred to as dyslipidemia [25]. The present findings showed that the GDM rats had low HDL levels and increased fat contents. Nevertheless, the administration of linagliptin to the GDM rats successfully controlled the alterations in their lipid levels. These findings corroborate the report of Zhou, et al. [26], on the positive effects of linagliptin on dyslipidemia in GDM rats.
The imbalance of cellular oxidants and antioxidants in favor of oxidants, resulting in a disturbance of redox signaling and/or molecular damage, is known as oxidative stress. Reactive oxygen species (ROS) are the byproducts of oxygen reduction. Reactive nitrogen species (RNS), in addition to ROS, significantly affect redox biology and consequently redox imbalance [27]. Several literature have demonstrated that hyperinsulinemia can increase the accumulation of free radicals and trigger oxidative stress damage to numerous organs [28]. During the gestational period, oxidative stress is considered low-grade or physiological since pregnancy has a high demand for oxygen from the mother, fetus, and placenta. The placenta, in turn, is an organ rich in mitochondria, resulting in greater production of reactive oxygen-nitrogen species (RONS) [6]. Despite its important physiological role, especially during pregnancy, the excessive production of RONS can override the antioxidant defense system, contributing to oxidative damage and thus causing considerable harm to women with GDM, from cell injury to death [29]. The present findings showed an increased MDA level while diminishing antioxidants like GSH, SOD, and CAT levels in the GDM rats. Interestingly, linagliptin administration considerably reduced the MDA level and enhanced the antioxidant levels in the GDM rats and this aligned with Shen et al [30], on the antioxidant efficacy of linagliptin to ameliorate the oxidative stress progression in diabetes.
Inflammatory cytokines are associated with the progression of GDM. IL-1β and IL-6 both compromise insulin signaling and decrease glucose absorption in target tissues, contributing to insulin resistance and hyperglycemia [31]. The increased synthesis of inflammatory cytokines may be connected to insulin resistance. Previous research indicates a connection between increased production of inflammatory cytokines such as IL-1β, and IL-6 and insulin resistance along the course of GDM disease [32]. In line with Rathinam et al.'s findings [33], elevated inflammatory cytokines IL-6 and IL-1β were established in the GDM model. It has been demonstrated that linagliptin therapy has anti-inflammatory properties to lower inflammatory cytokines in pathological conditions [34]. However, linagliptin administration down-regulates the upsurge in the expression of cytokines in the GDM model. This revealed the anti-inflammatory potential of linagliptin by suppressing the free radical-induced inflammatory cytokines expression in GDM and improving insulin sensitivity for glucose metabolism.
The current findings showed linagliptin had anti-hyperglycemic, anti-dyslipidemic, and anti-oxidative potentials on GDM. Linagliptin could be used as a safe medication for the management of hyperglycemia during gestation. However, no literature has elucidated any side effect of linagliptin on fetal outcome and further research should focus on the fetal outcome.
Declarations
Authors’ contributions: FO and OO conceived the original idea, and designed and supervised the research. FO, DA, MO, OM, and GA performed the experiments with the support of FO. MO and DA analyzed the data and prepared the manuscript. FO and MO reviewed the manuscript. All authors have read and approved the final manuscript.
Competing interests: No competing interests.
Funding: No Funding received from the Institution or Financial Organization
- Adam S, McIntyre HD, Tsoi KY, Kapur A, Ma RC, Dias S, et al. FIGO Committee on the Impact of Pregnancy on Long-term Health and the FIGO Division of Maternal and Newborn Health. Pregnancy as an opportunity to prevent type 2 diabetes mellitus: FIGO Best Practice Advice. International Journal of Gynaecology and Obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 2023; 160:56–67. Available from: https://doi.org/10.1002/ijgo.14537
- Omazić J, Viljetić B, Ivić V, Kadivnik M, Zibar L, Müller A, Wagner J. Early markers of gestational diabetes mellitus: what we know and which way forward? Biochemia Medica, 2021; 31(3):030502. Available from: https://doi.org/10.11613/bm.2021.030502
- Modzelewski R, Stefanowicz-Rutkowska MM, Matuszewski W, Bandurska S, Tankiewicz EM. Gestational Diabetes Mellitus-Recent Literature Review. Journal of clinical medicine, 2022; 11(19):5736. Available from: https://doi.org/10.3390/jcm11195736
- Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomedicine and pharmacotherapy = Biomedicine and pharmacotherapy, 2021; 143:112183. Available from: https://doi.org/10.1016/j.biopha.2021.112183
- 5. Yang X, Yang C, Lu W. Berberine Suppresses Gestational Diabetes in Streptozotocin-induced Diabetes Mellitus Rats by Suppression of Inflammatory Mediators. Ind. J. Pharm. Edu. Res, 2023; 57(2):423-431.
- de Mendonça E, Fragoso MB, de Oliveira JM, Xavier JA, Goulart MO, de Oliveira AC. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants. 2022; 11(1):129. Available from: https://doi.org/10.3390/antiox11010129
- Basu J, Datta C, Chowdhury S, Mandal D, Mondal NK, Ghosh A. Gestational Diabetes Mellitus in a Tertiary Care Hospital of Kolkata, India: Prevalence, Pathogenesis and Potential Disease Biomarkers. Exp Clin Endocrinol Diabetes. 2020; 128:216–223. Available from: https://doi.org/10.1055/a-0794-6057
- Wang J, Zhu QW, Cheng XY, Sha CX, Cui YB. Clinical significance of neutrophil-lymphocyte ratio and monocyte–lymphocyte ratio in women with hyperglycemia. Postgrad Med. 2020; 132:702–708. Available from: https://doi.org/10.1080/00325481.2020.1764235
- Camelo CW, Boggess K, Stürmer T, Brookhart MA, Benjamin DK, Jonsson FM. Association of Adverse Pregnancy Outcomes with Glyburide vs Insulin in Women with Gestational Diabetes. JAMA Pediatr: 2015; 169(18):1971-1977. Available from: https://doi.org/10.1001/jamapediatrics.2015.74
- Deacon CF, Holst JJ. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert opinion on investigational drugs, 2010;19(1):133-140. Available from: https://doi.org/10.1517/13543780903463862
- Klein T, Fujii M, Sandel J, Shibazaki Y, Wakamatsu K, Mark M, Yoneyama H. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Medical Molecular Morphology, 2014; 47:137-149. Available from: https://doi.org/10.1007/s00795-013-0053-9
- Rosenstock J, Marx N, Neubacher D, Seck T, Patel S, Woerle HJ, et al. Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events. Cardiovascular Diabetology. 2015; 14(1). Available from: https://doi.org/10.1186/s12933-015-0215-2
- Tradjenta [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; 2011
- Akinlade, O.M., Owoyele, B.V. and Soladoye, A.O. Streptozotocin-induced type 1 and 2 diabetes in rodents: a model for studying diabetic cardiac autonomic neuropathy. African Health Scences.2021; 21(2):719-727. https://doi.org/10.4314/ahs.v21i2.30
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6):499-502. Available from: https://pubmed.ncbi.nlm.nih.gov/4337382/
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes care, 41, S13–S27. Available from: https://doi.org/10.2337/dc18-s002
- Zhou L, Zhang R, Yang S, Zhang Y, Shi D. Astragaloside IV alleviates placental oxidative stress and inflammation in GDM mice. Endocrine Connections, 2020; 9(9):939–945. Available from: https://doi.org/10.1530/ec-20-0295
- Yu B, Liu Z, Fu Y, Wang Y, Zhang L, Cai Z, Yu F, Wang X, Zhou J, Kong W. CYLD Deubiquitinates Nicotinamide Adenine Dinucleotide Phosphate Oxidase 4 Contributing to Adventitial Remodeling. Arteriosclerosis, thrombosis, a vascular biology, 2017; 37(9):1698–1709. Available from: https://doi.org/10.1161/atvbaha.117.309859
- Wani K, Sabico S, Alnaami AM, Al-Musharaf S, Fouda MA, Turkestani IZ, Al-Ajlan A, et al. Early-pregnancy metabolic syndrome and subsequent incidence in gestational diabetes mellitus in Arab women. Frontiers in Endocrinology. 2020; 11: 98. Available from: https://doi.org/10.3389/fendo.2020.00098
- Abdel Aziz SM, Ahmed OM, Abd EL-Twab SM, Al-Muzafar HM, Amin KA, Abdel-Gabbar M. Antihyperglycemic Effects and Mode of Actions of Musa paradisiaca Leaf and Fruit Peel Hydroethanolic Extracts in Nicotinamide/Streptozotocin-Induced Diabetic Rats. Evidence-based Complementary and Alternative Medicine: 2020; 9276343. Available from: https://doi.org/10.1155/2020/9276343
- Siddiqui N, Ali J, Parvez S, Zameer S, Najmi AK, Akhtar M. Linagliptin, a DPP-4 inhibitor, ameliorates Aβ (1−42) peptides induced neuro-degeneration and brain insulin resistance (BIR) via insulin receptor substrate-1 (IRS-1) in rat model of Alzheimer’s disease. Neuropharmacology. 2021; 195:108662. Available from: https://doi.org/10.1016/j.neuropharm.2021.108662
- Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes, Obesity, and Metabolism. 2011; 13(3):258–67. Available from: https://doi.org/10.1111/j.1463-1326.2010.01350.x
- Bohn MK, Adeli K. Physiological and metabolic adaptations in pregnancy: importance of trimester-specific reference intervals to investigate maternal health and complications. Critical reviews in clinical laboratory sciences, 2022; 59(2):76–92. Available from: https://doi.org/10.1080/10408363.2021.1978923
- Zeljkovic A, Vekic J, Spasic S, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V, Gojkovic, T., Ardalic, D., Mandic-Markovic, V., Cerovic, N. and Mikovic, Z. Changes in LDL and HDL subclasses in normal pregnancy and associations with birth weight, birth length and head circumference. Maternal and Child Health Journal, 2013; 17(3): 556-565. Available from: https://doi.org/10.1007/s10995-012-1031-x
- Reis SA, Conceição LL, Rosa DD, Siqueira NP, Peluzio MCG. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutrition research reviews, 2017; 30(1), 36–49. Available from: https://doi.org/10.1017/s0954422416000226
- Zhou Y, Zhang X, Guo Y, Alarfaj AA, Liu J. Eupatilin mitigates Gestational diabetes in streptozotocin-induced diabetic pregnant rats through the regulation of inflammation and oxidative stress. Heliyon, 2024; 10(10): e30911.Available from: https://doi.org/10.1016/j.heliyon.2024.e30911
- Al-Shehri SS. Reactive oxygen and nitrogen species and innate immune response. Biochimie, 2021; 181:52–64. Available from: https://doi.org/10.1016/j.biochi.2020.11.022
- Zhang C, Yang Y, Chen R, Wei Y, Feng Y, Zheng W, Liao H, Zhang Z. Aberrant expression of oxidative stress-related proteins affects the pregnancy outcome of gestational diabetes mellitus patients. American journal of translational research, 2019; 11(1): 269–279. Available from: https://pubmed.ncbi.nlm.nih.gov/30787985/
- Duan Y, Sun F, Que S, Li Y, Yang S Liu G. Prepregnancy maternal diabetes combined with obesity impairs placental mitochondrial function involving Nrf2/ARE pathway and detrimentally alters metabolism of offspring. Obes. Res. Clin. Pract. 2018; 12(2), 90–100. Available from: https://doi.org/10.1016/j.orcp.2017.01.002
- Shen Z, Yang C, Zhu P, Tian C, Liang A. Protective effects of syringing against oxidative stress and inflammation in diabetic pregnant rats via TLR4/MyD88/NF-κB signaling pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 2020; 131: 110681. Available from: https://doi.org/10.1016/j.biopha.2020.110681
- Ma Y, Xu S, Meng J, Li L. Protective effect of nimbolide against streptozotocin induced gestational diabetes mellitus in rats via alteration of inflammatory reaction, oxidative stress, and gut microbiota. Environ. Toxicol. 2022; 37:1382-1393. Available from: https://doi.org/10.1002/tox.23491
- Li Y, Xi H, Zhang H. Protective effect of sinomenine against inflammation and oxidative stress in gestational diabetes mellitus in female rats via TLR4/ MyD88/NF κB signaling pathway. J. Food Biochem. 2021; 45, e13952.
- Ayyasamy Rathinam, Leelavinothan Pari, Manigandan Venkatesan, Shankar Munusamy. Myrtenal attenuates oxidative stress and inflammation in a rat model of streptozotocin-induced diabetes. Archives of Physiology and Biochemistry, 2019. Available from: https://doi.org/10.1080/13813455.2019.1670212
- Ide M, Sonoda N, Inoue T, Kimura S, Minami Y, Makimura H, et al. The dipeptidyl peptidase-4 inhibitor, linagliptin, improves cognitive impairment in streptozotocin-induced diabetic mice by inhibiting oxidative stress and microglial activation. PLoS ONE. 2020; 15(2): e0228750. Available from: https://doi.org/10.1371/journal.pone.0228750