More Information
Submitted: July 15, 2024 | Approved: July 19, 2024 | Published: July 22, 2024
How to cite this article: Girsh E. Disposable Diapers in Infancy and Their Potential Detrimental Impact on Male Fertility in Adulthood. Clin J Obstet Gynecol. 2024; 7(3): 084-092. Available from: https://dx.doi.org/10.29328/journal.cjog.1001170
DOI: 10.29328/journal.cjog.1001170
Copyright License: © 2024 Girsh E. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Disposable Diapers in Infancy and Their Potential Detrimental Impact on Male Fertility in Adulthood
Eliezer Girsh*
ELNAT Reproduction, Rehovot 76241, Israel
*Address for Correspondence: Eliezer Girsh, ELNAT Reproduction, Rehovot 76241, Israel, Email: [email protected]
The overall human fertility rate has been continuously declining across the globe for a number of reasons. This review summarizes data, which proposes that the use of disposable diapers for newborns and infants may incur reproductive harm in adulthood. More than 70 years ago, a disposable synthetic waterproof baby diaper was developed, mainly to reduce the burden of working mothers. Modern diapers feature the same original design, which contains one unit of disposable material wrapped around the perineum to collect urine and feces. This design results in an increase in internal area temperatures by 2-4 °C, which can be detrimental to the function and development of reproductive cells. Moreover, the standard diaper template promotes the free passage of feces, including fecal bacteria, to the genitals, which can lead to urogenital infection and reproductive impairments. The available clinical data suggest that diaper use during infancy may have a negative impact on fertility after puberty. There is a critical need for additional studies to better assess the impact of diapers on reproductive health.
The global fertility rate is continuously declining [1,2]. There are multiple factors that can have adverse effects on the human reproductive system, some of which are general, e.g., environmental or lifestyle, while others are more specific, e.g., genetic or epigenetic. Environmental risk factors include food and air pollution [3,6], and diurnal and seasonal periods [7]. Similarly, it was suggested that lifestyles, such as excessive/vigorous physical activity or sedentary behavior [8,9], occupation, and stress [10,13], could be harmful to fertility. Age, medical conditions, genetic diseases, and epigenetics can also negatively impact fertility [14,22]. Recently, it was proposed that the lack of natural selection among humans has been matched by a decline in reproductive functions [23].
Over 70 years ago, the first disposable synthetic, waterproof baby diaper was developed and marketed. The main impetus for this development was the mobilization of a female workforce during World War II, leaving women with little time or energy to manage the enormous task of washing cotton diapers at home. Today, baby diapers are in widespread use across the world. The early disposable diaper comprised cellulose as the main absorbent material and a plastic outer sheet to prevent messy leakage. Apart from improvements in absorbency, textile, and grip over time, there have been no conceptual changes in the product design/template. In fact, modern diapers share the same original, convenient, and easy-to-use, simple template, which contains a single unit of disposable material wrapped around the perineum to collect urine and feces. Recently, it was found that diapers increase the temperature of the genital area by 2°C - 4 °C [24,25]. Moreover, the standard diaper styling allows for free passage of feces, including fecal bacteria, to the genitals, which can lead to acute or chronic urogenital infection. The present article proposes that the use of disposable diapers for newborns and infants can be detrimental to the child’s health and reproduction functions in adulthood.
Maintaining testicular temperature
The optimal temperature range for normal spermatogenesis is 32°C - 34 ℃ [26], which is the average temperature range in the scrotum. Yet, the average body temperature is 36.4 ℃ and can rise to 38 ℃ or more in case of illness. High body temperature has been shown to negatively affect sperm quality [27,28], with the severity of testicular harm dependent on the duration and amplitude of the fever [29]. The lower temperature of the scrotum is achieved mainly by the external position of the testicles. Testicular cooling and warming are both controlled by the tunica dartos and the cremasteric muscles. The tunica dartos muscle forms the lining of the scrotum and relaxes when exposed to high temperatures, which leads to movement of the testes farther away from the body, resulting in its subsequent cooling. The cremasteric muscle is located in the spermatic cord and is also involved in moving the testes farther forward from the body. The scrotal sac is also involved in testicular thermoregulation, by contributing to heat loss through evaporative cooling [30]. An additional mechanism involved in counter-current heat exchange involves the pampiniform venous plexus which removes and diverts heat from arriving warm arterial blood to the cooler venous blood leaving the testes [31-34]. When cooled, the scrotum muscles contract, which then draws the testicles closer to the body, preventing hypothermia. The negative impact of high body or scrotum temperature on male fertility is well established [28,35]. High scrotal temperatures also increase the risk of testicular cancer [36]. These data underscore the importance of scrotal ventilation and maintenance of the physiological scrotal temperature.
Heat stress in adult testicles
Failure to cool to the natural scrotal temperature has been associated with impaired spermatogenesis, low sperm count, poor sperm motility, and abnormal morphology in ejaculation [37]. Lifestyle, occupational, environmental, and pathophysiological factors contribute to increased scrotal temperature range. A major outcome of heat stress on the testis is apoptotic destruction of germ cells [38], a bulk for further spermatogenesis. A 1 ℃ increase in testicular temperature results in a more than 14% drop in spermatogenesis [39,40]. Hyperthermic exposure to spermatozoa impacts spermatozoa count, morphology, aneuploidy [41], and DNA integrity and triggers apoptosis [42,43], which results in poor fertilization capacity [44]. Chronically elevated scrotal temperature can lead to testicular germinal atrophy, spermatogenic arrest, and aneuploidy of sperm cells [41]. Testicular heating mostly affects spermatocytes and round spermatids in adulthood [27,45,46] and has therefore been proposed as a method of male contraception [47-50].
In a sitting position, the scrotum comes into contact with the body, which can increase the scrotal temperature to body temperature in a short time [51,52]. Indeed, significantly higher than average testicular temperature was measured in wheelchair-bound paraplegic men with spinal cord injuries [53]. In line with the above, the incidence of pathospermia among men with sit-down jobs, such as professional drivers, is significantly higher, as compared to other professionals, with increased prevalence in proportion to the number of years of driving [54] and delayed conception [55]. Similarly, scrotal temperature was 1.5°C - 2 ℃ higher in clothed as compared to naked men [56]. Further, sperm concentration gradually decreased after three months in men who regularly wore tight-fitting clothes and gradually increased after a change to loose-fitting clothing [57]. Also, the improvement of sperm motility and sperm morphology was statistically insignificant after three months by nocturnal scrotal cooling [58] with a positive trend of improved male fertility [59]. Taken together, these studies underscore the role of genital heat stress in impaired semen quality.
Heat stress affects spermatogenesis also in an indirect way via testicular somatic cells. Spermatogenesis is supported by somatic Sertoli cells in the seminiferous tubule and by somatic steroidogenic Leydig cells in the interstitial space of the testicle.
Contacting Sertoli cells form the blood-testis barrier (BTB) by various types of cell junctions [60]. The BTB is responsible for the anchoring of spermatogonia to Sertoli cells, and the differentiation of mature spermatogonia to primary spermatocytes [61,62]. As such, a functional BTB is obligatory for optimal spermatogenesis. The number of Sertoli cells in the human testis increases sharply during the first 3 months of life, stays stable until the start of puberty with a further increase during puberty, and then gradually increases to adult values after 25 years of age [63]. Transient heating of the testis results in a broad range of changes in Sertoli cell function [64,65]. A reduction in the number of Sertoli cells in mice was noted one month following a single, 30-minute exposure to 42 ℃ [66]. Increasing the temperature to 37 ℃ inhibited Androgen Binding Protein (ABP) production by Sertoli cells [67]. Microscopic examination of the rat testis after heat exposure showed mitochondrial degeneration, dilatation of the smooth endoplasmic reticulum, and wider spaces between Sertoli cells [68], all of which adversely impact normal spermatogenesis.
The somatic steroidogenic Leydig cells exhibit dynamic development and are responsible for testosterone production, Sertoli cell development, and BTB function. The number of fetal-type Leydig cells reaches a maximum in the 15th week of pregnancy and is still appreciable at birth. These cells disappear at about 3-6 months of age. Thereafter, the number of Leydig cells begins to increase and reaches a plateau at approximately 1-8 years of age. These cells then disappear and are replaced by prepubertal-type followed by mature adult-type cells [69-71]. Serum and testicular testosterone concentrations are also appreciable up to about 6 months of age, after which, they drop to low levels until puberty [70,71]. Steroidogenic Acute Regulatory Protein (StAR) gene expression and testicular testosterone concentrations declined in the 48 h after scrotal insulation in bulls [72], indicating a severe impact of heat stress on steroidogenesis in Leydig cells.
Heat-stressed cell apoptosis
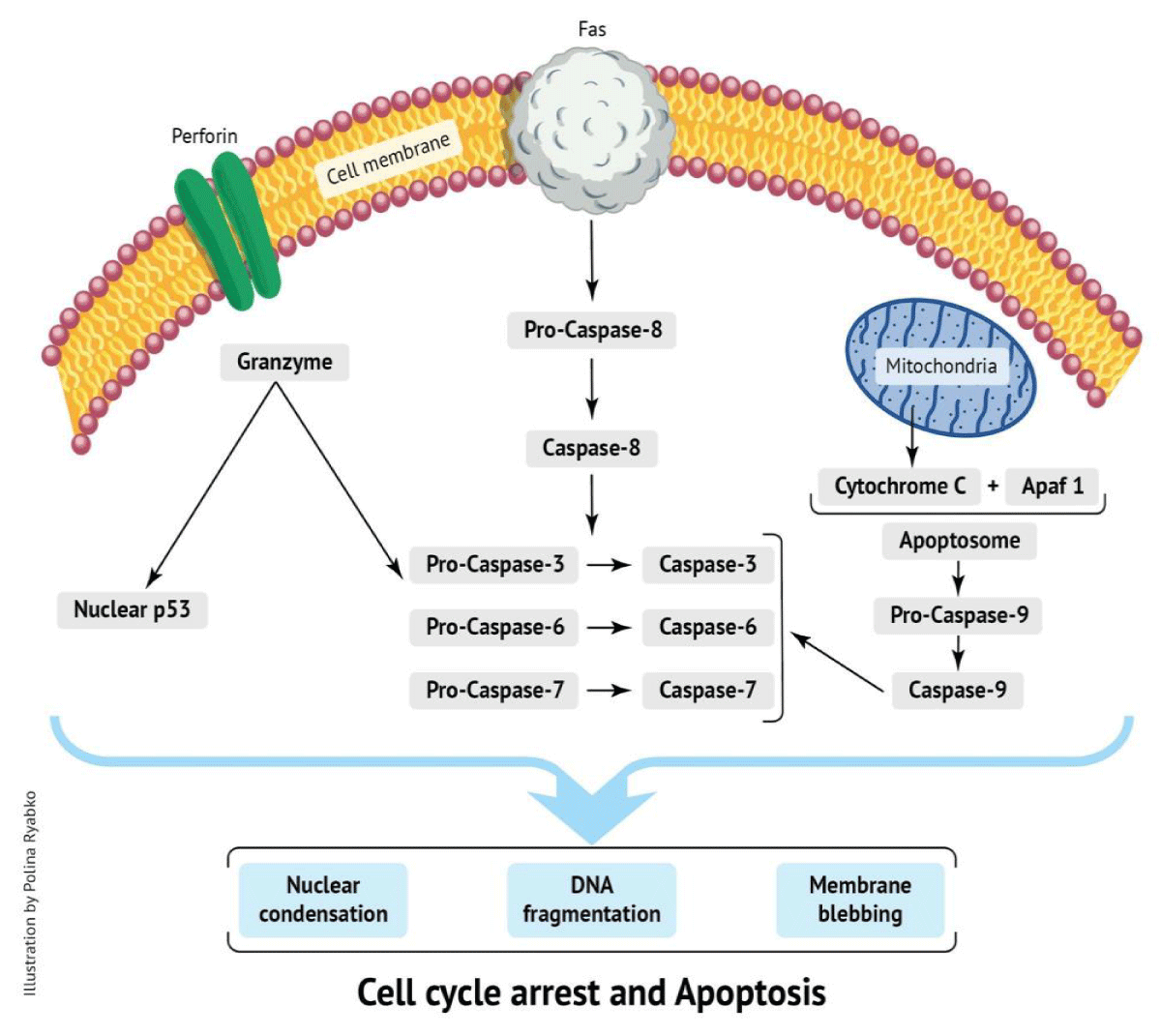
Cell apoptosis can be induced by many triggers, with temperature being a strong inducer of the process [73]. Heat-stressed Sertoli and spermatogenic cells undergo apoptosis [74,75]. Generally, there are two major apoptotic pathways: the extrinsic/death receptor pathway (receptor Fas – cytosolic caspase 3 pathway) and the intrinsic/mitochondrial pathway (cytochrome-c –caspase 3 pathway) [76] (Figure 1).
Figure 1: Effect of stress on testicular functions.
Molecules of one pathway can be influenced by molecules in the other [77]. Additional cell death pathways include the perforin/granzyme pathway [78] and the tumor suppressor p53 pathway [79]. The Fas and Fas ligand (FasL) system is also involved in germ cell apoptosis in humans. For example, hypospermatogenesis, such as in cases of maturation arrest and Sertoli cell-only syndrome, is a result of a Fas/FasL-mediated process. Patients with post-meiotic germ cell arrest show increased Fas expression in germ cells, suggesting that the Fas/FasL system is involved in gamete apoptosis [80].
Possible adverse effects of diaper usage on fertility
As spermatocytes and round spermatids are not present in infancy [81], the topic of spermatogenesis in childhood has not received due attention, and the effect of high genital temperature during infancy on fertility in adulthood has not yet been studied.
Babies are often sedentary or asleep for long periods and diapers become a heat trap. Indeed, it was shown that cotton and synthetic diapers increase the scrotal temperature by 2 °C and 3 °C, respectively [24,25]. A significant increase in the scrotal/testicular temperature was observed in male neonates (0-4 weeks), infants (1-12 months), and toddlers (1-4.5 years) who wore plastic as opposed to cotton diapers [24]. However, there is no data regarding the effect of increased scrotal temperature in infancy on reproductive functions after puberty and in adulthood.
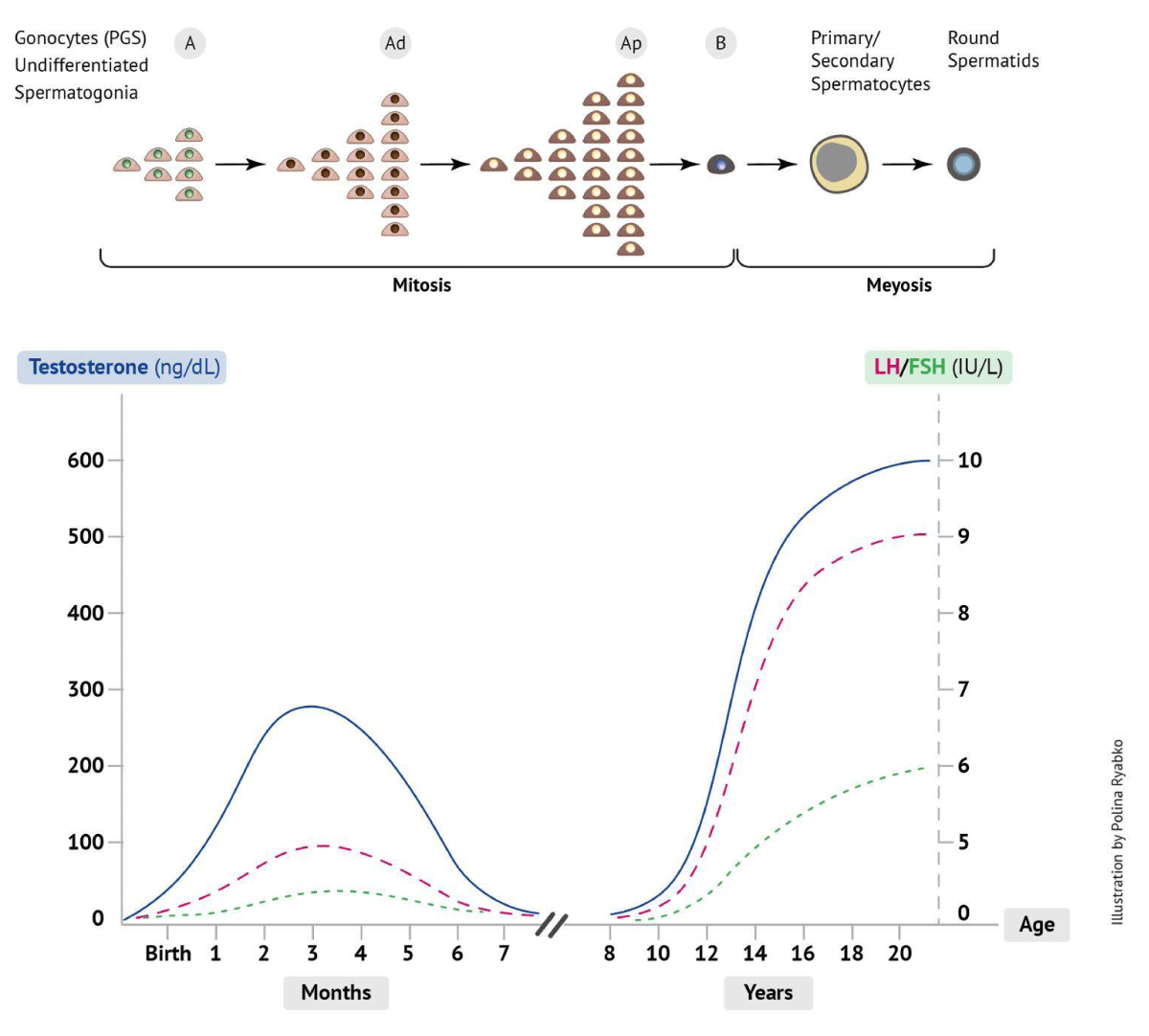
In human males, there are three endocrine stages of "puberty maturation" that are dependent on testosterone secretion from the developing testes prior to real puberty. The first wave of testosterone is observed during embryonic development (10-24 weeks of gestation) and affects the development of the male reproductive system. The second wave of testosterone normally occurs at the age of 2-3 months and is associated with the active mitotic proliferation and transformation of gonocytes (fetal reproductive stem cells) into the A dark (Ad) spermatogonia (Figure 2). This period is characterized by densely stained chromatin of Ad spermatogonia, which later shifts to the pale A (Ap) spermatogonia with granular chromatin, and later to mature type B spermatogonia, which forms a self-mitotic-replicating pool (warehouse) of spermatogonial stem cells that will contribute to future spermatogenesis [82-84]. During these periods, mitotic gonocytes and spermatogonia are vulnerable to heat stress [85-87].
Figure 2: Endocrine axis and spermatogenesis at neonate, infancy, and puberty.
As proliferation and transformation of spermatogonia occurs in the first period of life, associated with high levels of testosterone, differentiation of spermatogonia into primary spermatocytes begins later, at the age of 3-5 years [88], when the level of testosterone is low. While male germ cells undergo extensive mitosis during fetal and postnatal life, meiotic division begins at puberty. The third wave of testosterone occurs between the ages of 9 and 14 years and persists until approximately age 50, after which testosterone levels slowly decline [89]. The endocrine activity at different ages affects testicular development. The testis of boys < 1 year of age weighs about 0.5 g, and there is a slow increase in weight until the age of 10-14 years when each testis weighs about 1.5 g. Between ages 14 and 18 years, the testes rapidly increase in size to about 12 g each and later to an adult size of 20-25 g [90]. This testicular development is driven by the proliferation of all types of testicular cells, including gonocytes and somatic cells, and is driven by endocrine activity. It also was noted that endocrine activity during infancy is associated with proliferation but not maturation of the Sertoli and germ cells, whereas during puberty both proliferation and maturation occur [63]. Lower testicular volume and sperm count were observed in monkeys subjected to reversible blockade of the pituitary-gonadal axis during early postnatal life [92]. Considering the above, it can be postulated that spermatogenesis in adulthood is directly influenced by events in infancy. This phenomenon is well described in cryptorchid patients.
Cryptorchidism is a well-known infant pathology, in which one or both testicles do not descend into the scrotum after birth, and remain at different levels of the inguinal canal. Failed testicular descent into the scrotum before the age of 6 months has been associated with infertility [92]. This can be explained by the impaired transition of gonocytes into type Ad spermatogonia in cryptorchid testes [93], which has been associated with spermatogenic arrest [94]. Indeed, undescended testicles contain a significantly lower number of Ad spermatogonia, which is a high risk for subsequent infertility, as compared to descended testes [95-97]. In line with the above, infants with low Ad stem cell counts later showed 25 times lower spermatozoa count as compared to men with normal numbers of Ad stem cells in infancy [98]. One of the explanations for infertility in cryptorchidism is the negative effect of body temperature stress on the proliferation and differentiation of spermatogonia in the neonate and infant testicles [99,100].
Currently, there is growing evidence that the scrotum requires a low physiological temperature in the neonatal period as well. Taken together, diaper use during infancy may have a negative impact on fertility after puberty.
Possible effect of fecal infections on fertility
The genital tract is not sterile and features a unique microbiome that exists in symbiosis with the body. The most common bacteria in the vaginal microbial community are lactobacilli (L. iners, L. jensenii, L. helveticus) [101], which maintain a local pH, rendering it difficult for other, external microorganisms to develop and grow. When contaminated, for example by fecal microorganisms, the balance of the microbiome is disrupted. This can lead to an acute inflammatory process of the genital and urinary tracts, followed by chronic inflammation.
Bacterial families of fecal origin are divided into six major groups: Prevotellaceae, Ruminococcaceae, Lachnospiraceae, Enterobacteriaceae, Moraxellaceae, and Aeromonadaceae, which include well-known genera and species, such as Ruminicoccus, Clostridium, Bifidobacterium, Helicobacter, Streptococcus, Mycobacterium, Actinomycetes, Campylobacter, Escherichia coli, Enterococcus fecalis, and others. Some of these genera (Enterococcus, Enterobacter, Helicobacter, Campylobacter) are resistant to antibiotics.
In both disposable and reusable diapers, feces, especially in a soft state or liquefied by urine, easily spread to the front compartment of the diaper, providing access to fecal flora to the genitals and urethral tract. Typically, half of infected infants are asymptomatic [102]. At neonatal age and early infancy, babies cannot yet verbalize the feeling of pain or discomfort. The only way to express discomfort is by crying, the cause of which is not always clear to adults. This discomfort can be triggered by bacterial infection and inflammation, mostly of fecal origin, e.g., E. coli and E. faecalis.
Urinary Tract Infections (UTI) are one of the most common bacterial infections in infants under 1 year of age [103-106]. E. coli is the most frequent bacterial pathogen responsible for UTI, accounting for 85% - 90% of cases [107], followed by Enterococcus faecalis [105]. However, asymptomatic colonization of the urinary tract can occur and may be missed in as many as 50% of infants who need to get to primary care [108]. Among infants presenting with fever, the overall prevalence of UTI was 7% [103]. Before the age of 1 year, the incidence of UTI in boys is higher than in girls. Shaikh et al. found a 7.5% and 20.1% incidence of febrile UTI in female and male infants less than three months of age, respectively [103]. However, after 1 year, girls are much more likely to develop a UTI than boys [109].
The number of infants treated for UTI is steadily increasing [110,111]. For example, in 2013, aggregate hospital charges for inpatient UTI management exceeded 630 million dollars [112]. UTI in children is of concern because it can be associated with acute mortality (i.e., urosepsis) and/or chronic medical complications, such as renal scarring, hypertension, chronic renal insufficiency, and pre-eclampsia [111,113,114]. Approximately 10% of young infants with UTI have concomitant bacteremia. A study conducted in 7 Houston (TX, USA) day-care centers found that 19% of diapered children were colonized with resistant E. coli [115]. It was suggested that decreased frequency of diaper changes allows more time for urinary and fecal bacteria to colonize the peri-urethral area, leading to infection [116].
Endometriosis is a common benign gynecological condition affecting 15% of women at reproductive ages and is associated with chronic pain and reduced quality of life [117]. The etiology and clinical course of endometriosis are not yet clear [118]. Recently, it was suggested that genital, intestinal, and oral microbiota influence the development of endometriosis [119]. It was shown that microorganisms of fecal origin, such as E. coli and Enterococcus could be involved in endometriosis and uterine infection by shifting the intravaginal pH to higher than 4.5 [120,121]. Increased presence of Enterobacteriaceae, Streptococcaceae, and Staphylococaceae bacteria, alongside a decrease in the normal flora of Lactobacillaceae, were detected in endometrial swab and cervical mucus samples from women with endometriosis [122,123]. A difference in the distribution of the microbiota from the lower to the upper reproductive tract has been shown in patients with endometriosis, with a gradual microbiota increase and spreading up the reproductive tract [124].
Microbiological analysis detected asymptomatic genital tract infection in more than 40% of in vitro fertilization (IVF) couples [125]. Analysis of genital swabs from females failing IVF showed the presence of Enterococcus faecalis, Escherichia coli, and Streptococcus agalactiae. In sharp contrast, 86% of couples with successful IVF outcomes showed microbiologically negative results [125]. In line with this, there are no data regarding the effect of fecal contamination of the genital tract in infancy and childhood, and inflammation of the endometrium on female reproductive functions in adulthood. In male IVF patients, increases in sperm cell DNA fragmentation, and decreases in sperm cell concentration, motility, progressive motility, and protamine composition have been associated with bacteriospermia, partly of intestinal origin [126]. The same work showed that the fertilization rate of oocytes by sperm cells from patients with bacteriospermia (Staphylococcus, Escherichia coli, Enterococcus faecalis, Streptococcus agalactiae) was significantly reduced compared to patients without bacteriospermia. Enterococcus faecalis was the most prevalent species in bacteriospermia-positive semen samples and was found to negatively affect both sperm cell morphology and motility [125]. Both E. coli and E. faecalis are the most frequent pathogens in chronic prostatic infections [127]. The relationship between this pathology in adulthood and fecal contamination of the genitals and urinary tract during infancy and childhood remains to be verified.
Testicular thermoregulation is of great importance to ensure the production of viable spermatozoa and to maintain fertility. Failure to regulate scrotal temperatures or exposure to high temperatures results in testicular heat stress, apoptosis, and testicular hypofunction. Understanding the mechanisms of testicular heat stress can help guide preventive actions and the development of targeted male infertility therapies. Future studies on the effect of bacterial fecal contamination of the genital tract in infancy and childhood on reproductive health in adulthood will be of great importance. The accumulated evidence suggests that the standard diaper template may have a negative effect on male and female health and reproductive functions both in childhood and adulthood. Therefore, further investigation of the health implications of the current diaper template is warranted. Recommendations on the measures to be taken on the use of disposable diapers may include reducing as much as possible the utilization of diapers both in daily use and age and leaving neonates, infants, and toddlers without diapers as much as possible at every opportunity. The development of a new diaper design could potentially help treat newborns more naturally and possibly partially prevent infertility in adulthood.
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609-613. Available from: https://doi.org/10.1136/bmj.305.6854.609.
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan ShH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646-659. Available from: https://doi.org/10.1093/humupd/dmx022.
- Ramlau-Hansen CH, Thulstrup AM, Aggerholm AS, Jensen MS, Toft G, Bonde JP. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum Reprod. 2007;22(1):188-196. Available from: https://doi.org/10.1093/humrep/del364.
- Ramlau-Hansen CH, Thulstrup AM, Olsen J, Ernst E, Andersen CY, Bonde JP. Maternal smoking in pregnancy and reproductive hormones in adult sons. Int J Androl. 2008;31(6):565-572. Available from: https://doi.org/10.1093/humrep/del364.
- Afeiche M, Williams PL, Mendiola J, Gaskins AJ, Jørgensen N, Swan SH, et al. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod. 2013;28(8):2265-2275. Available from: https://doi.org/10.1093/humrep/det133.
- Bieniek JM, Kashanian JA, Deibert CM, Grober ED, Lo KC, Brannigan RE, et al. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertil Steril. 2016;106(5):1070-1075. Available from: https://doi.org/10.1016/j.fertnstert.2016.06.041.
- Hjollund NHI, Bonde JPE, Jensen TK, Olsen J. Diurnal scrotal skin temperature and semen quality. Int J Androl. 2000;23:309-318. Available from: https://doi.org/10.1046/j.1365-2605.2000.00245.x.
- Gaskins AJ, Afeiche MC, Hauser R, Williams PL, Gillman MW, Tanrikut C, et al. Paternal physical and sedentary activities in relation to semen quality and reproductive outcomes among couples from a fertility center. Hum Reprod. 2014;29(11):2575-2582. Available from: https://doi.org/10.1093/humrep/deu212.
- Gaskins AJ, Mendiola J, Afeiche MC, Jørgensen N, Swan SH, Chavarro JE. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med. 2015;49(4):265-270. Available from: https://doi.org/10.1136/bjsports-2012-091644.
- Xia Y, Cheng S, Bian Q, Xu L, Collins MD, Chang HC, et al. Genotoxic effects on spermatozoa of carbaryl-exposed workers. Toxicol Sci. 2005;85(1):615-623. Available from: https://doi.org/10.1093/toxsci/kfi066.
- Abu-Musa AA, Nassar AH, Hannoun AB, Usta IM. Effect of the Lebanese civil war on sperm parameters. Fertil Steril. 2007;88(6):1579-1582. Available from: https://doi.org/10.1016/j.fertnstert.2007.01.067.
- Hannoun AB, Nassar AH, Usta IM, Zreik TG, Abu-Musa AA. Effect of war on the menstrual cycle. Obstet Gynecol. 2007;109(4):929-932. Available from: https://doi.org/10.1097/01.aog.0000257170.83920.de.
- Nargund VH. Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12(7):373-382. Available from: https://doi.org/10.1038/nrurol.2015.112.
- Gosden R, Trasler J, Lucifero D, Faddy M. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet. 2003;361(9373):1975-1977. Available from: https://doi.org/10.1016/S0140-6736(03)13592-1.
- Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19(7):1548-1553. Available from: https://doi.org/10.1093/humrep/deh304.
- Girsh E, Katz N, Genkin L, Girtler O, Bocker J, Bezdin S, et al. Male age influences oocyte-donor program results. J Assist Reprod Genet. 2008;25(4):137-143. Available from: https://doi.org/10.1007/s10815-008-9215-4.
- Shiraishi M, Haruna M, Matsuzaki M, Ota E, Murayama R, Murashima S. Association between the serum folate levels and tea consumption during pregnancy. Biosci Trends. 2010;4(5):225-230. Available from: https://www.biosciencetrends.com/article/346.
- Richard-Eaglin A. Male and Female Hypogonadism. Nurs Clin North Am. 2018;53(3):395-405. Available from: https://doi.org/10.1016/j.cnur.2018.04.006.
- Santoro A, Chianese R, Troisi J, Richards S, Nori SL, Fasano S, et al. Neuro-toxic and reproductive effects of BPA. Curr Neuropharmacol. 2019;17(12):1109-1132. Available from: https://doi.org/10.2174/1570159x17666190726112101.
- Chen W, Bai MZ, Yang Y, Sun D, Wu S, Sun J, et al. ART strategies in Klinefelter syndrome. J Assist Reprod Genet. 2020;37(9):2053-2079. Available from: https://doi.org/10.1007/s10815-020-01818-2.
- Saget S, Kappeler L, Grandjean V, Leneuve P, Berthaut I, Faure C, et al. Association between metabolic disorders and seminal plasma miRNA levels: a pilot study. Basic Clin Andro. 2022;32(1):9. Available from: https://doi.org/10.1186/s12610-022-00159-7.
- Bendarzka-Czerwińska A, Zmarzły N, Morawiec E, Panfil A, Bryś K, Czarniecka J, Ostenda A, et al. Endocrine disorders and fertility and pregnancy: An update. Front Endocrinol (Lausanne). 2023. Available from: https://doi.org/10.3389/fendo.2022.970439.
- Girsh E. Infertility: What is our direction? Austin Andrology. 2018;3(1):1020. Available from: https://www.refaelcare.com/wp-content/uploads/2019/01/Girsh-andrology-2018.pdf.
- Partsch CJ, Aukamp M, Sippell WG. Scrotal temperature is increased in disposable plastic lined nappies. Arch Dis Child. 2000;83(4):364-368. Available from: https://doi.org/10.1136/adc.83.4.364.
- Grove GL, Grove MJ, Bates NT, Wagman LM, Leyden JJ. Scrotal temperatures do not differ among young boys wearing disposable or reusable diapers. Skin Res Technol. 2002;8(4):260-270. Available from: https://doi.org/10.1034/j.1600-0846.2002.00336.x.
- Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl. 1995;18:169-184. Available from: https://doi.org/10.1111/j.1365-2605.1995.tb00408.x.
- Carlsen E, Andersson A-M, Petersen JH, Skakkebaek NE. History of febrile illness and variation in semen quality. Hum Reprod. 2003;18(10):2089-2092. Available from: https://doi.org/10.1093/humrep/deg412.
- Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril. 2007;88:970.e1-7. Available from: https://doi.org/10.1016/j.fertnstert.2006.12.045.
- Paul C, Murray AA, Spears N, Saunders PT. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction. 2008;136:73-84. Available from: https://doi.org/10.1530/REP-08-0036.
- Waites G, Voglmayr J. The functional activity and control of the apocrine sweat glands of the scrotum of the ram. Aust J Agric Res. 1963;14:839-851. Available from: https://www.publish.csiro.au/cp/ar9630839.
- Harrison RJ, J.S. Weiner JS. Vascular patterns of the mammalian testis and their functional significance. J Exp Biol. 1949;26:304-316. Available from: https://doi.org/10.1242/jeb.26.3.304.
- Dahl E, Herrick J. A vascular mechanism for maintaining testicular temperature by counter-current exchange. Surg Gynecol Obstet. 1959;108:697-705. Available from: https://pubmed.ncbi.nlm.nih.gov/13659355/.
- Lazarus B, A.W. Zorgniotti AW. Thermoregulation of the human testis. Fertil Steril. 1975;26:757-759. Available from: https://pubmed.ncbi.nlm.nih.gov/1157962/.
- Glad Sorensen H, Lambrechtson J, Einer-Jensen N. Efficiency of the counter current transfer of heat and 113Xenon between the pampiniform plexus and testicular artery of the bull under in-vitro conditions. Int J Androl. 1991;14:232-240. Available from: https://doi.org/10.1111/j.1365-2605.1991.tb01085.x.
- Zhang MH, Zhang AD, Shi ZD, Wang LG, Qiu Y. Changes in levels of seminal nitric oxide synthase, macrophage migration inhibitory factor, sperm DNA integrity, and caspase-3 in fertile men after scrotal heat stress. PLoS ONE. 2015;10(10). Available from: https://doi.org/10.1371/journal.pone.0141320.
- Karagas MR, Weiss NS, Strader CH, Daling JR. Elevated intrascrotal temperature and the incidence of testicular cancer in noncryptorchid men. Am J Epidemiol. 1989;129(6):1104-1109. Available from: https://doi.org/10.1093/oxfordjournals.aje.a115232.
- Durairajanayagam D, Sharma RK, du Plessis SS, Agarwal A. Testicular heat stress and sperm quality. In: du Plessis SS, Agarwal A, Sabanegh Jr. ES, editors. Male Infertility: A Complete Guide to Lifestyle and Environmental Factors. Springer, New York; 2014;105–125. Available from: https://link.springer.com/chapter/10.1007/978-1-4939-1040-3_8.
- Kim B, Park K, Rhee K. Heat stress response of male germ cells. Cell Mol Life Sci. 2013;70:2623-2636. Available from: https://doi.org/10.1007/s00018-012-1165-4.
- Wang C, McDonald V, Leung A, Superlano L, Berman N, Hull NL, et al. Effect of increased scrotal temperature on sperm production in normal men. Fertil Steril. 1997;68:334-339. Available from: https://doi.org/10.1016/s0015-0282(97)81525-7.
- Hjollund NHI, Storgaard L, Ernst E, Bonde JP, Olsen J. Impact of diurnal scrotal temperature on semen quality. Reprod Toxicol. 2002;16:215-221. Available from: https://doi.org/10.1016/S0890-6238(02)00025-4.
- Abdelhamid MHM, Esquerre-Lamare C, Walschaerts M, Ahmad G, Mieusset R, Hamdi S, Bujan L. Experimental mild increase in testicular temperature has drastic, but reversible, effect on sperm aneuploidy in men: A pilot study. Reprod Biol. 2019;19:189-194. Available from: https://doi.org/10.1016/j.repbio.2019.06.001.
- Perez-Crespo M, Pintado B, Gutierrez-Adan A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev. 2008;75:40-47. Available from: https://doi.org/10.1002/mrd.20759.
- Rao M, Xia W, Yang J, Hu L, Hu S, Lei H, et al. Transient scrotal hyperthermia affects human sperm DNA integrity, sperm apoptosis, and sperm protein expression. Andrology. 2016;4:1054-1063. Available from: https://doi.org/10.1111/andr.12228.
- Yaeram J, Setchell BP, Maddocks S. Effect of heat stress on the fertility of male mice in vivo and in vitro. Reprod Fertil Dev. 2006;18:647-653. Available from: https://doi.org/10.1071/rd05022.
- Chowdhury AK, Steinberger E. Early changes in the germinal epithelium of rat testes following exposure to heat. J Reprod Fertil. 1970;22:205-212. Available from: https://doi.org/10.1530/jrf.0.0220205.
- Kandeel FR, Swerdloff RS. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril. 1988;49:1-23. Available from: https://doi.org/10.1016/s0015-0282(16)59640-x.
- Voegeli M. Contraception through temporary male sterilization. Smith College Library, Northampton, MA; 1956.
- Mieusset R, Grandjean H, Mansat A, Pontonnier F. Inhibiting effect of artificial cryptorchidism on spermatogenesis. Fertil Steril. 1985;43(4):589-594. Available from: https://doi.org/10.1016/s0015-0282(16)48502-x.
- Shafik A. Contraceptive efficacy of polyester-induced azoospermia in normal men. Contraception. 1992;45:439-451. Available from: https://doi.org/10.1016/0010-7824(92)90157-o.
- Mieusset R, Bujan L. The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Inter J Androl. 1994; 17:186-191. https://doi.org/10.1111/j.1365-2605.1994.tb01241.x.
- Bujan L, Daudin M, Charlet JP, Thonneau P, Mieusset R. Increase in scrotal temperature in car drivers. Hum Reprod. 2000;15:1355-1357. Available from: https://doi.org/10.1093/humrep/15.6.1355.
- Hjollund NHI, Storgaard L, Ernst E, Bonde JP, Olsen J. The relation between daily activities and scrotal temperature. Reprod Toxicol. 2002;16:209-214. Available from: https://doi.org/10.1016/s0890-6238(02)00026-6.
- Brindley GS. Deep scrotal temperature and the effect on it of clothing, air temperature, activity, posture and paraplegia. Br J Urol. 1982;54:49-55. Available from: https://doi.org/10.1111/j.1464-410x.1982.tb13510.x.
- Sas M, Szöllösi J. Impaired spermiogenesis as a common finding among professional drivers. Arch Androl. 1979;3:57-60. Available from: https://doi.org/10.3109/01485017908985049.
- Thonneau P, Bujan L, Multigner L, Mieusset R. Occupational heat exposure and male fertility: a review. Hum Reprod. 1998;13:2122-2125. Available from: https://doi.org/10.1093/humrep/13.8.2122.
- Mieusset R, Bengoudifa B, Bujan L. Effect of posture and clothing on scrotal temperature in fertile men. J Androl. 2007;28:170-175. Available from: https://doi.org/10.2164/jandrol.106.000646.
- Sanger WG, Friman PC. Fit of underwear and male spermatogenesis: a pilot investigation. Reprod Toxicol. 1990;4:229-232. Available from: https://doi.org/10.1016/0890-6238(90)90063-2.
- Jung A, Leonhardt F, Schill WB, Schuppe HC. Influence of the type of undertrousers and physical activity on scrotal temperature. Hum Reprod. 2005;20:1022-1027. Available from: https://doi.org/10.1093/humrep/deh697.
- Nikolopoulos I, Osman W, Haoula Z, Jayaprakasan K, Atiomo W. Scrotal cooling and its benefits to male fertility: A systematic review. J Obstet Gynecol. 2013;33:338-342. Available from: https://doi.org/10.3109/01443615.2012.758088.
- Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012; 64:16-64. https://doi.org/10.1124/pr.110.002790
- Wu S, Yan M, Ge R, Cheng CY. Crosstalk between Sertoli and germ cells in male fertility. Trends Mol Med. 2020;26(2):215-231. Available from: https://doi.org/10.1016/j.molmed.2019.09.006.
- Nguyen HT, Martin LJ. Classical cadherins in the testis: how are they regulated? Reprod Fertil Dev. 2023;35(14):641-660. Available from: https://doi.org/10.1071/RD23084.
- Cortes D, Muller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl. 1987;10:589-596. Available from: https://doi.org/10.1111/j.1365-2605.1987.tb00358.x.
- Zhang X-S, Zhang Z-H, Jin X, Wei P, Hu X-Q, Chen M, et al. Dedifferentiation of adult monkey Sertoli cells through activation of extracellularly regulated kinase 1/2 induced by heat treatment. Endocrinology. 2006;147(3):1237-1245. Available from: https://doi.org/10.1210/en.2005-0981.
- Li L, Han Z-Y, Li C-M, Jiang X-Q, Wang G-L. Upregulation of heat shock protein 32 in Sertoli cells alleviates the impairments caused by heat shock-induced apoptosis in mouse testis. Cell Stress Chaperones. 2013;18(3):333-351. Available from: https://doi.org/10.1007/s12192-012-0385-8.
- Reid BO, Mason KA, Withers HR, West J. Effects of hyperthermia and radiation on mouse testis stem cells. Cancer Res. 1981;41:4453-4457. Available from: https://pubmed.ncbi.nlm.nih.gov/7306969/.
- Hagenas L, Ritzen EM, Svensson J, Hansson V, Purvis K. Temperature dependence of Sertoli cell function. Int J Androl. 1978;1:449-458. Available from: https://doi.org/10.1111/j.1365-2605.1978.tb00501.x.
- Kanter M, Aktas C, Erboga M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: an immunohistochemical and ultrastructural study. Toxicol Ind Health. 2013;29:99-113. Available from: https://doi.org/10.1177/0748233711425082.
- Nistal M, Paniagua R, Regadera J, Santamaria L, Amat P. A quantitative morphological study of human Leydig cells from birth to adulthood. Cell Tissue Res. 1986;246:229-236. Available from: https://doi.org/10.1007/BF00215884 .
- Chemes HE. Leydig cell development in humans; in Payne AH, Hardy MP, Russell LD (eds): The Leydig Cell. Vienna, Cache River Press. 1996;175-202. Available from: https://cir.nii.ac.jp/crid/1572824500854487296.
- Zirkin BR, Papadopoulos V. Leydig cells: formation, function, and regulation. Biol Reprod. 2018;99(1):101-111. Available from: https://doi.org/10.1093/biolre/ioy059.
- Rizzoto G, Ferreira J, Codognoto V, Oliveira K, Mogollon Garcia H, Pupulim AGR, et al. Testicular hyperthermia reduces testosterone concentrations and alters gene expression in testes of Nelore bulls. Theriogenology. 2020;152:64-68. Available from: https://doi.org/10.1016/j.theriogenology.2020.04.029.
- Yamamoto Y, Hishikawa D, Ono F. Trpv4-mediated apoptosis of Leydig cells induced by high temperature regulates sperm development and motility in zebrafish. Commun Biol. 2024;7:96. Available from: https://doi.org/10.1038/s42003-023-05740-y.
- Zhang ZH, Jin X, Zhang XS, Hu ZY, Zou RJ, Han CS, et al. Bcl-2 and Bax are involved in experimental cryptorchidism-induced testicular germ cell apoptosis in rhesus monkey. Contraception. 2003;68:297-301. Available from: https://doi.org/10.1016/s0010-7824(03)00176-8.
- Cai H, Qin D, Peng S. Responses and coping methods of different testicular cell types to heat stress: overview and perspectives. Biosci Rep. 2021;41(6). Available from: https://doi.org/10.1042/BSR20210443.
- Shasha Ch, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Phil Trans R Soc B. 2010;365:1501-1515. Available from: https://doi.org/10.1098/rstb.2009.0124.
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277-288. Available from: https://doi.org/10.1038/nrc776.
- Martinvalet D, Zhu P, Lieberman J. Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity. 2005 22:355-370. Available from: https://doi.org/10.1016/j.immuni.2005.02.004.
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307-310. Available from: https://doi.org/10.1038/35042675.
- Francavilla S, D’Abrizio P, Cordeschi G, Pelliccione F, Necozione S, Ulisse S, et al. Fas expression correlates with human germ cell degeneration in meiotic and post-meiotic arrest of spermatogenesis. Mol Hum Reprod. 2002;8:213-220. Available from: https://doi.org/10.1093/molehr/8.3.213.
- Hilscher W. The genetic control and germ cell kinetics of the female and male germ line in mammals including man. Hum Reprod. 1991;6:1416-1425. Available from: https://doi.org/10.1093/oxfordjournals.humrep.a137281.
- Simorangkir DR, Marshall GR, Ehmcke JJ, Schlatt S, Plant TM. Prepubertal expansion of dark and pale type A spermatogonia in the Rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol Reprod. 2005;73:1109-1115. Available from: https://doi.org/10.1095/biolreprod.105.044404.
- Kubota H, Brinster RL. Spermatogonial stem cells. Biol Reprod. 2018;99(1):52-74. Available from: https://doi.org/10.1093/biolre/ioy077.
- Diao L, Turek PJ, John CM, Fang F, Reijo Pera RA. Roles of spermatogonial stem cells in spermatogenesis and fertility restoration. Front Endocrinol (Lausanne). 2022; Available from: https://doi.org/10.3389/fendo.2022.895528.
- Nakamura M, Namiki M, Okuyama A, Matsui T, Doi Y, Takeyama M, et al. Temperature sensitivity of human spermatogonia and spermatocytes in vitro. Arch Androl. 1987;19(2):127-132. Available from: https://pubmed.ncbi.nlm.nih.gov/3435194/.
- Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol. 2012;19:538-550. Available from: https://doi.org/10.1111/j.1442-2042.2012.02982.x.
- Gao WJ, Li HX, Feng J, Lu XR, Yin PL, Jia H, et al. Transcriptome analysis in high temperature inhibiting spermatogonial stem cell differentiation in vitro. Reprod Sci. 2023;30(6):1938-1951. Available from: https://doi.org/10.1007/s43032-022-01133-4.
- Hess A, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. In: Yan Cheng C, editor. Molecular Mechanisms in Spermatogenesis. Springer; 2008. Available from: https://link.springer.com/chapter/10.1007/978-0-387-09597-4_1.
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724-731. Available from: https://doi.org/10.1210/jcem.86.2.7219.
- Müller J, Skakkebaek NE. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int J Androl. 1983;6:143-158. Available from: https://doi.org/10.1111/j.1365-2605.1983.tb00333.x.
- Mann DR, Gould KG, Collins DC, Wallen K. Blockade of neonatal activation of the pituitary-testicular axis: effects on peripubertal luteinizing hormone and testosterone secretion and on testicular development in monkeys. J Clin Endocrinol Metab. 1989;68:600-607. Available from: https://doi.org/10.1210/jcem-68-3-600.
- Hadziselimovic F, Herzog B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet. 2001;358:1156-1157. Available from: https://doi.org/10.1016/s0140-6736(01)06274-2.
- Kamisawa H, Kojima Y, Mizuno K, Imura M, Hayashi Y, Kohri K. Attenuation of spermatogonial stem cell activity in cryptorchid testes. J Urol. 2012;187:1047-1052. Available from: https://doi.org/10.1016/j.juro.2011.10.170.
- Holstein AF, Roose-Runge EC, Schirren C. Illustrated Pathology of Human Spermatogenesis. Grosse, Berlin. 1988. Available from: https://cir.nii.ac.jp/crid/1130282271845626112.
- Lipshultz LI. Cryptorchidism in the subfertile male. Fertil Steril. 1976;27:609-620. Available from: https://doi.org/10.1016/s0015-0282(16)41889-3.
- Hadziselimovic F, Hoecht B. Testicular histology related to fertility outcome and postpubertal hormone status in cryptorchidism. Klin Padiatr. 2008;220(5):302-307. Available from: https://doi.org/10.1055/s-2007-993194.
- Zivkovic D, Varga J, Konstantinidis G, Vlaski J, Snyder HM, Hadziselimovic F. Regional differences in maturation of germ cells of cryptorchid testes: role of environment. Acta Paediatr. 2009;98:1339-1343. Available from: https://doi.org/10.1111/j.1651-2227.2009.01325.x
- Docampo MJ, Hadziselimovic F. Molecular pathology of cryptorchidism-induced infertility. Sex Dev. 2015;9(5):269-278. Available from: https://doi.org/10.1159/000442059 .
- Yin Y, Stahl BC, DeWolf WC, Morgentaler A. P53 and Fas are sequential mechanisms of testicular germ cell apoptosis. J Androl. 2002;23:64-70. Available from: https://doi.org/10.1002/jand.2002.23.1.64.
- Zhang X, Yuan JX, Liu T, Lue YH, Jin X, Tao SX, et al. Expression of orphan receptors TR2, TR3, TR4, and p53 in heat-treated testis of cynomolgus monkeys (Macaca fascicularis). J Androl. 2006;27:405-413. https://doi.org/10.2164/jandrol.05165
- Ursell LK, Gunawardana M, Chang S, Mullen M, Moss JA, Herold BC, et al. Comparison of the vaginal microbial communities in women with recurrent genital HSV receiving acyclovir intravaginal rings. Antiviral Res. 2014;102:87-94. Available from: https://doi.org/10.1016/j.antiviral.2013.12.004.
- Gill MA, Schutze GE. Citrobacter urinary tract infections in children. Pediatr Infect Dis J. 1999; 18(10):889-892. Available from: https://doi.org/10.1097/00006454-199910000-00010.
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood. A meta-analysis. Pediatr Infect Dis J. 2008;27:302-308. Available from: https://doi.org/10.1097/inf.0b013e31815e4122.
- Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. Available from: https://doi.org/10.1097/INF.0000000000000225.
- Shrestha LB, Baral R, Poudel P, Khanal B. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr. 2019;19(1):36. Available from: https://doi.org/10.1186/s12887-019-1410-1.
- Hum S, Liu H, Shaikh N. Risk factors for the development of febrile recurrences in children with a history of urinary tract infection. J Pediatr. 2022;243:152-157. Available from: https://doi.org/10.1016%2Fj.jpeds.2021.12.037.
- Becknell B, Schober M, Korbel L, Spencer D. The diagnosis, evaluation and treatment of acute and recurrent pediatric urinary tract infections. Expert Rev Anti Infect Ther. 2015;13(1):81-90. Available from: https://doi.org/10.1586/14787210.2015.986097.
- Zorc JJ, Levine DA, Platt SL, Dayan PS, Macias CG, Krief W, et al. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics. 2005;116:644-648. Available from: https://doi.org/10.1542/peds.2004-1825.
- Hellström A, Hanson E, Hansson S, Hjälmas K, Jodal U. Association between urinary symptoms at 7 years old and previous urinary tract infection. Arch Dis Child. 1991;66:232-234. Available from: https://doi.org/10.1136/adc.66.2.232.
- Freedman AL. Urologic diseases in North America Project: trends in resource utilization for urinary tract infections in children. J Urol. 2005;173(3):949-954. Available from: https://doi.org/10.1097/01.ju.0000152092.03931.9a.
- Spencer JD, Schwaderer A, McHugh K, Hains DS. Pediatric urinary tract infections: an analysis of hospitalizations, charges, and costs in the USA. Pediatr Nephrol. 2010;25(12):2469-2475. Available from: https://doi.org/10.1007/s00467-010-1625-8.
- Healthcare Cost and Utlization Project Kids’ Inpatient Database. 2013.
- El-Khatib M, Packham DK, Becker GJ, Kincaid-Smith P. Pregnancy-related complications in women with reflux nephropathy. Clin Nephrol. 1994;41:50-55. Available from: https://pubmed.ncbi.nlm.nih.gov/8137569/.
- Zhang Y, Bailey R. A long term follow up of adults with reflux nephropathy. N Z Med J. 1995;108:142-144. Available from: https://pubmed.ncbi.nlm.nih.gov/7761049/.
- Reves RR, Murray BE, Pickering LK, Prado D, Maddock D, Bartlett AV. Children with trimethoprim and ampicilin-resistant fecal Escherichia coli in day-care centers. J Infect Dis. 1987;156:758-762. Available from: https://doi.org/10.1093/infdis/156.5.758.
- Waseem Y, Naseeb WM, Hamza M. Diapers, an underestimated cause of urinary tract infections in children. Stud Corner Let. Editor 2018) 68(7):1151. Available from: https://pubmed.ncbi.nlm.nih.gov/30317331/.
- De Graaf AA, D’Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L; WERF EndoCost Consortium. Simoens S. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28:2677-2685. Available from: https://doi.org/10.1093/humrep/det284.
- Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177-200. Available from: https://doi.org/10.1016/j.bpobgyn.2004.01.007.
- Sobstyl A, Chalupnik A, Mertowska P, Grywalska E. How do microorganisms influence the development of endometriosis? Participation of genital, intestinal and oral microbiota in metabolic regulation and immunopathogenesis of endometriosis. Int J Mol Sci. 2023;24(13):10920. Available from: https://doi.org/10.3390/ijms241310920.
- Khan KN, Kitajima M, Hiraki K, Yamaguchi N, Katamine S, Matsuyama T, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. 2010;94:2860-2863. Available from: https://doi.org/10.1016/j.fertnstert.2010.04.053.
- Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosis. Hum Reprod. 2014;29:2446-2456. Available from: https://doi.org/10.1093/humrep/deu222
- Khan KN, Fujishita A, Masumoto H, Muto H, Kitajima M, Masuzaki H, et al. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:69-75. Available from: https://doi.org/10.1016/j.ejogrb.2016.01.040.
- Akiyama K, Nishioka K, Khan KN, Tanaka Y, Mori T, Nakaya T, et al. Molecular detection of microbial colonization in cervical mucus in women with and without endometriosis. Am J Reprod Immunol. 2019;82. Available from: https://doi.org/10.1111/aji.13147.
- Wei W, Zhang X, Tang H, Zeng L, Wu R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann Clin Microbiol Antimicrob. 2020;19:15. Available from: https://doi.org/10.1186/s12941-020-00356-0.
- Ricci S, De Giorgi S, Lazzeri E, Luddi A, Rossi S, Piomboni P, et al. Impact of asymptomatic genital tract infections on in vitro fertilization (IVF) outcome. PLoS One. 2018;13(11). Available from: https://doi.org/10.1371/journal.pone.0207684.
- Zeyad A, Hamad MF, Hammadeh ME. The effects of bacterial infection on human sperm nuclear protamine P1/P2 ratio and DNA integrity. Andrologia. 2018;50(20). Available from: https://doi.org/10.1111/and.12841.
- Stamatiou K, Magri V, Perletti G, Papadouli V, Recleiti N, Mamali V, et al. Chronic prostatic infection: microbiological findings in two Mediterranean populations. Arch Ital Urol Androl. 2019;91(3):177-181. Available from: https://dx.doi.org/10.4081/aiua.2019.3.177.