More Information
Submitted: April 15, 2024 | Approved: April 29, 2024 | Published: April 30, 2024
How to cite this article: Kotigala DSK, Adedipe TO. Morular Metaplasia of the Endometrium: A Case Report and Literature Review: Care Pathways based on Molecular Biology. Clin J Obstet Gynecol. 2024; 7: 059-062.
DOI: 10.29328/journal.cjog.1001165
Copyright License: © 2024 Kotigala DSK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Endometrial metaplasia; Morular metaplasia; Atypical endometrial hyperplasia; Endometrioid carcinoma; Conservative treatment; Fertility
Morular Metaplasia of the Endometrium: A Case Report and Literature Review: Care Pathways based on Molecular Biology
Dhanushka SK Kotigala and Tolu O Adedipe*
Department of Obstetrics and Gynaecology, Women and Children's Hospital, Hull University Teaching Hospital NHS Trust, Hull
*Address for Correspondence: Tolu O Adedipe, Department of Obstetrics and Gynaecology, Women and Children's Hospital, Hull University Teaching Hospital NHS Trust, Hull, Email: [email protected]
Background: Endometrial morular metaplasia, a clinical conundrum from a diagnostic and management angle given its rarity and low oncogenic potential, has been linked to endometrial hyperplasia and carcinoma.
Case report: A 77-year-old woman with no significant past medical history, was found to have an asymptomatic thickened endometrium on pelvic imaging, after presenting with lower abdominal pain, 3yrs ago. Diagnostic hysteroscopy identified an endometrial polyp within a pyometra. Histopathology showed focal complex endometrial hyperplasia without atypia with superimposed morular metaplasia(EMM) with a negative microbiology assay.
Following conservative management with multidisciplinary team(MDT) overview, as-per patient choice with 6-monthly follow-up hysteroscopy, endometrial biopsies and a short use of the Mirena® Intrauterine system (discontinued following poor tolerance), histopathology shows resolved hyperplasia with persistent EMM. Due to persistent disease, a hysterectomy is under consideration.
Discussion: Current evidence suggests that a sub-type of EMM, a likely histological manifestation of beta-catenin (CTNNB1) gene mutation: could be a precursor of endometrial hyperplasia and low-grade endometrioid-endometrial carcinoma sub-type. Though low-grade in nature, the increased recurrence risk raises significant concerns.
Prognostication following gene mutation identification can help with management options which include conservative, hormonal therapy with adjunct repeat endometrial sampling: or hysterectomy. The optimal frequency of endometrial sampling when uterine-sparing, is unclear, leading to a management conundrum, whilst persistent disease may require a hysterectomy..
Conclusion: Management of endometrial morular metaplasia can be difficult but must reflect the woman’s choice with a MDT-overview. Immuno-histochemical tools utilizing new molecular biological advances, can simplify the diagnostic and prognostication processes, aiding clinical management.
The term metaplasia stands for the process of replacing one type of differentiated somatic cells to another differentiated somatic cell type in the same tissue [1]. When it comes to endometrium it is the transformation of usual endometrial phenotype into other types of Mullerian epithelial differentiation [2]. Morular metaplasia (EMM) is a rare and distinctive kind of epithelial metaplasia commonly seen in endometrium [2,3]. Generally, it is placed under the category of squamous metaplasia [2], other types of epithelial metaplasia being mucinous, tubal, eosinophilic, papillary, secretory and hobnail [2].
Pathological features
EMM is mulberry-like [4], rounded-to-polygonal syncytial nests of bland, immature, squamous cells that fill the lumen of the endometrial glands [2]. Although EMM is considered a variation of squamous endometrial metaplasia, unlike ‘conventional’ squamous differentiation (SD), it lacks the characteristic intercellular bridges, distinctive cell membranes and keratinization featured by squamous metaplasia [3]. But some researchers argue that it appears as the primitive form of distinctive type of SD founded on the immunohistochemical studies over mixed EMM/SD lesions [3].
The term ‘morulosis’ denotes a much more extensive form, affecting entire endometrium or a large area of it as opposed to more localized version of EMM [4]. Intriguingly, morules and morule-like features have been reported in uterine endometrioid carcinomas and other extra-uterine tumours [5] as well. A case report after obtaining consent, is described below.
A 77-year-old woman with no significant past medical history, presented initially 3 years ago, with lower abdominal pain which was investigated via pelvic ultrasound scan. A diagnostic hysteroscopy to investigate the incidental thickened endometrium with no associated post-menopausal bleeding, identified an endometrial polyp within a pyometra. Histopathology showed focal complex endometrial hyperplasia without atypia with superimposed morular metaplasia with a negative microbiology. Immunohistochemistry for p53 was negative.
Conservative management with multidisciplinary team (MDT) overview, as-per patient choice with 6 monthly follow-up hysteroscopy, endometrial biopsy and a short use of the Mirena® Intra uterine system (discontinued due to poor tolerance). Follow-up biopsies indicates persistent morular metaplasia whilst the hyperplasia has since resolved. Though currently conservatively being managed, a consideration is for a hysterectomy is underway, due to the discomfort at repeated endometrial sampling. This emphasizes the role of treatment morbidity on patient choice.
Aetiology: Molecular biological basis for pathogenesis
Usually, changes in environment trigger metaplastic changes1. Changes in the internal milieu of endometrium and uterine cavity, such as hyperoestrogenism, inflammation, repeated irritation or endometrial polyps [2] can potentially stimulate endometrial metaplasia. In particular, endometrial squamous metaplasia is often thought to be secondary to prolonged irritation6 which can be either mechanical as in case of intrauterine device or previous intrauterine surgical interventions like curettage, or inflammatory, for example chronic endometritis and pyometria [2].
Yet, on the contrary, EMM is linked to mutational phenomenon [6] and more commonly associated with complex endometrial glandular lesions, atypia and endometrioid adenocarcinoma [2,7] which is another distinguishing feature when compared with conventional SD which usually progresses to primary squamous cell carcinoma rather than adenocarcinoma [2].
Nakatani, et al. in 2002 first suggested that morula formation is secondary to beta-catenin gene (CTNNB1) mutation based on studies over the specimens of low-grade adenocarcinoma of the fetal lung (L-FLAC)/ well-differentiated fetal adenocarcinoma (WDFA) which were also characterized by morules8 also supported by Litta, et al. [7]. Beta-catenin is a transcriptional activator of the WNT signaling pathway which promote cell proliferation and differentiation, therefore beta-catenin gene (CTNNB1) is considered a proto-oncogene. Mutation of this gene and resultant malfunctioning give rise to the aberrant intra-nuclear localization of beta-catenin rather than its usual location on the cell membrane [7]. This is well demonstrated by immunohistochemistry where morules typically exhibit higher nuclear beta-catenin immunoreactivity [7].
This observation also applies to endometrial morular metaplasia [9]. On the other hand, well-differentiated glandular epithelium only exhibits membrane-bound beta-catenin [7]. Furthermore, unlike normal endometrium, morules were negative for oestrogen and progesterone receptors, and thus hormonally inert [10].
Gauging the malignant potential
Better insight about carcinogenesis and the role of beta-catenin in this context will enable the clinicians to handle cases of EMM with utmost care. Carcinogenesis involves accumulation of genetic mutations over a period of time of which oncogenes hold a major role along with tumor suppressor and mismatch-repair genes. Gain-of-function mutation of proto-oncogene CTNNB1 which is now called an oncogene leads to aberrant WNT/beta catenin signaling activity which facilitates cancer stem cell renewal, cell proliferation, and differentiation. This is considered one of the early events which culminates in to carcinogenesis [11], which is an indispensable fact when predicting the risk of malignant transformation and hence determining the threshold of operative intervention.
Saegusa, et al. in their study in 2001, compared beta-catenin localization in cells in normal, hyperplastic and endometrioid carcinoma with normal samples having highest immune-reactivity score for beta-catenin at the membrane and decreasing steadily through non-atypical hyperplasia, atypical hyperplasia, carcinoma. However, with increasing beta-catenin nuclear localization the pattern was the opposite with carcinoma showing highest nuclear localization [12].
Buttressing this hypothesis, ovarian and endometrial endometrioid carcinomas characterized by morules were seen to carry higher nuclear localization of beta-catenin [13]. Adding emphasis to this observation, Kim and Jeong in their study in 2019 found a high frequency of CTNNB1 (beta-catenin gene) mutations in endometrial (16%), hepatobiliary (12%), melanoma (7%), and colorectal (6%) cancers [14].
Ming-Chieh Lin, et al. [15] noted histological appearance of morule-associated glands carried higher risk of progress in to carcinoma. On the other hand, they also found that all squamous morules were devoid of sex hormone receptors and had either undetectable or extremely low-proliferation rates in marked contrast to coexisting glandular component [15] which contradicted their aforementioned observation.
Even though beta-catenin gene mutation is associated with low-grade endometrioid endometrial carcinoma, Kurnit, et al. in 2017 found that such low-grade cancers harboring beta catenin gene mutation showed significantly decreased recurrence-free interval [16].
Therefore, from the prognosis point of view, it can be postulated that beta-catenin mutation and hence morular formation could be the starting point of a continuum of oncogenic pathway that could potentially give rise to low-grade endometrial cancer, yet with higher recurrence, thus needing careful follow-up if not early surgical intervention.
Immunohistochemical approach to improving diagnosis
These new molecular biological insights shed light on how to improve the diagnostic strategies and prognostication in relation to morular metaplasia. Along with the clinical utility of immunostaining to demonstrate higher nuclear concentration of beta-catenin, biomarkers such as CDX2 and CD10 have gained particular interest.
Chiarelli, et al. in 2006 proposed that higher CD10 positivity at cell membranes is a useful marker of the presence of morules, and helps to differentiate morules from background glandular components that appear negative [17].
In 2008, Houghton et al suggested that nuclear positivity of CDX2 is a useful marker of morular identification because of their consistent positivity for this marker and further stated that this feature could be attributed to over-expression of nuclear beta-catenin [18].
Given the clinical significance of beta-catenin mutation in typifying EMM in terms of possible malignant transformation, these novel diagnostic approaches could potentially be useful, particularly in dealing with endometrial specimens with poorer quality such as fragmented aspirative biopsies, moderately autolytic endometrial lesions and necrotic debris where identification of morules may be difficult. Of particular note, CD10 retained its positivity even in context of moderate autolysis and necrotic debris [18].
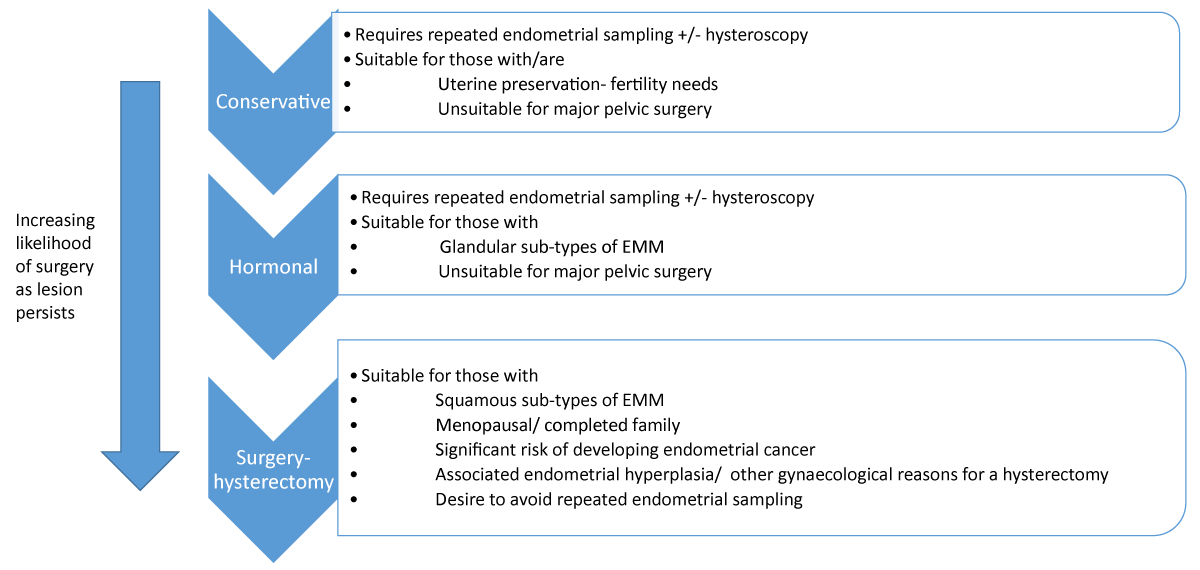
Management
Because of the rarity of the EMM, development of care pathways has always been a challenge therefore management which can vary from conservative, hormonal and surgical excision, has to take on board the below.
The indolent nature of the disease with poor mitotic activity, being hormonally inert and some association with only low-grade carcinoma warrants the room for conservative management.
However, the presence of well-established association of beta-catenin mutation with many endometrial and extra-uterine cancers, and the higher risk of recurrence of endometrial endometrioid carcinomas which shows morular metaplasia, would suggest a more aggressive treatment.
Thirdly, any concurrent pre-malignant conditions might incline management towards aggressive interventions whilst measures aiming at uterine preservation, will be largely borne from fertility needs.
Litta, et al. described 2 cases of successful disease resolution in women of reproductive-age who were successfully managed conservatively with follow-up hysteroscopies and resections but no progestogen therapy. Whilst one showed endometrial hyperplasia, the other one presented initially with atypical hyperplasia. The latter after no disease-progression over a 5-year follow-up period, underwent a successful physiological pregnancy [7].
Even though progestogen therapy has long been considered to be effective as a treatment modality for pre-cancerous, even atypical glandular endometrial lesions, Ming-Chieh Lin, et al. suggest it is not the case for EMM [15]. Instead, morules could be resistant to the apoptotic effects of progestins [15] which is supported by the fact that morules do not feature progesterone receptors and are hormonally inert. Progestogen can still be effective to induce regression of endometrial premalignant glandular lesions of endometrial premalignant lesion, as they further observed that morule-bearing endometrial intra-epithelial neoplasia (EIN) showed degenerative nuclear pyknosis and cytoplasmic shrinkage of the glandular, but not squamous (morular), components [14].
In the context of menopause or no need for uterine preservation for fertility needs, more radical surgical intervention following excision of the primary lesion, has a place given current evidence, we postulate. Particularly in the presence of other factors – multiple endometrial pathologies or co-existing gynaecological indications for major surgery. However patient choice must be respected as we found in this case where a preference was for regular sampling once the complex focal hyperplasia resolved in response to the short use of hormones, due to concerns about treatment morbidity of major surgery.
Undoubtedly the patient should be fully informed regarding current evidence alongside individual peri-operative risks prior to making any decisions for surgery. The importance of regular follow-up if they opt for uterine conservation (conservative approach) under a multidisciplinary team overview, should be emphasized. Due to the lack of a disease natural history timeline, the frequency of endometrial sampling for those unsuitable for hysterectomy or seeking uterine preservation is unclear, leading to a management conundrum. A factor noted in other case reports [19,20].
As there are few reported cases, there is limited evidence towards optimum generic management- prevention or therapeutic interventions, raising the need for an individualistic approach.
However we propose the management algorithm below:
Figure 1: Management algorithm.
Morular endometrial metaplasia is a rare, essentially benign, hormonally inert condition with proliferative activity; with associations with pre-malignant and malignant neoplastic changes.
Management of morular endometrial metaplasia can be difficult but must reflect the woman’s choice with a MDT overview. Immuno-histochemical tools utilizing new molecular biological advances, can simplify the diagnostic and prognostication processes which aid clinical management.
Of particular note, an EMM sub-type thought to arise from CTNNB1 (beta-catenin) gene mutation, is related to endometrioid endometrial carcinomas and other extra-uterine cancers. The higher recurrence risk of endometrial cancers which feature this mutation may warrant a hysterectomy as a treatment option in these women.
- Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017 Oct;17(10):594-604. doi: 10.1038/nrc.2017.68. Epub 2017 Sep 1. PMID: 28860646; PMCID: PMC5998678.
- Goyette ERJ, Bentz JL. Endometrial metaplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusmetaplasia.html. Accessed March 15th, 2024.
- Travaglino A, Raffone A, Gencarelli A, Raimondo D, Moretta P, Pignatiello S, Granata M, Seracchioli R, Zullo F, Insabato L. Relationship between morular metaplasia and squamous differentiation in endometrial carcinoma. Pathol Res Pract. 2021 Jan;217:153307. doi: 10.1016/j.prp.2020.153307. Epub 2020 Nov 30. PMID: 33316539.
- Kujdowicz M, Milewicz T, Adamek D. Extensive squamous metaplasia (morulosis) of the endometrium as a clinical and pathological problem: a case report and literature study. Gynecol Endocrinol. 2020 May;36(5):460-464. doi: 10.1080/09513590.2019.1696304. Epub 2020 Feb 10. PMID: 32037914.
- Makishi S, Kinjo T, Sawada S, Chinen K, Hirayasu T, Hamada T, Saito K, Iwamasa T. Morules and morule-like features associated with carcinomas in various organs: report with immunohistochemical and molecular studies. J Clin Pathol. 2006 Jan;59(1):95-100. doi: 10.1136/jcp.2005.026237. PMID: 16394288; PMCID: PMC1860262.
- Nicolae A, Preda O, Nogales FF. Endometrial metaplasias and reactive changes: a spectrum of altered differentiation. J Clin Pathol. 2011 Feb;64(2):97-106. doi: 10.1136/jcp.2010.085555. Epub 2010 Dec 1. PMID: 21126963.
- Litta P, Codroma A, D'Agostino G, Breda E. Morular endometrial metaplasia: review of the literature and proposal of the management. Eur J Gynaecol Oncol. 2013;34(3):243-7. PMID: 23967555.
- Nakatani Y, Masudo K, Miyagi Y, Inayama Y, Kawano N, Tanaka Y, Kato K, Ito T, Kitamura H, Nagashima Y, Yamanaka S, Nakamura N, Sano J, Ogawa N, Ishiwa N, Notohara K, Resl M, Mark EJ. Aberrant nuclear localization and gene mutation of beta-catenin in low-grade adenocarcinoma of fetal lung type: up-regulation of the Wnt signaling pathway may be a common denominator for the development of tumors that form morules. Mod Pathol. 2002 Jun;15(6):617-24. doi: 10.1038/modpathol.3880575. PMID: 12065775.
- Travaglino A, Raffone A, Russo D, Guadagno E, Pignatiello S, Moretta P, Zullo F, Del Basso De Caro M, Insabato L, Mascolo M. Does endometrial morular metaplasia represent odontogenic differentiation? Virchows Arch. 2021 Sep;479(3):607-616. doi: 10.1007/s00428-021-03060-2. Epub 2021 Mar 5. PMID: 33666744; PMCID: PMC8448715.
- Chiarelli S, Buriticá C, Litta P, Ciani S, Guarch R, Nogales FF. An immunohistochemical study of morules in endometrioid lesions of the female genital tract: CD10 is a characteristic marker of morular metaplasia. Clin Cancer Res. 2006 Jul 15;12(14 Pt 1):4251-6. doi: 10.1158/1078-0432.CCR-06-0398. PMID: 16857799.
- Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020 Dec 4;13(1):165. doi: 10.1186/s13045-020-00990-3. PMID: 33276800; PMCID: PMC7716495.
- Saegusa M, Hashimura M, Yoshida T, Okayasu I. beta- Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001 Jan;84(2):209-17. doi: 10.1054/bjoc.2000.1581. PMID: 11161379; PMCID: PMC2363713.
- Saegusa M, Okayasu I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001 May;194(1):59-67. doi: 10.1002/path.856. PMID: 11329142.
- Kim S, Jeong S. Mutation Hotspots in the β-Catenin Gene: Lessons from the Human Cancer Genome Databases. Mol Cells. 2019 Jan 31;42(1):8-16. doi: 10.14348/molcells.2018.0436. Epub 2019 Jan 7. PMID: 30699286; PMCID: PMC6354055.
- Lin MC, Lomo L, Baak JP, Eng C, Ince TA, Crum CP, Mutter GL. Squamous morules are functionally inert elements of premalignant endometrial neoplasia. Mod Pathol. 2009 Feb;22(2):167-74. doi: 10.1038/modpathol.2008.146. Epub 2008 Sep 19. PMID: 19180120; PMCID: PMC2633489.
- Kurnit KC, Kim GN, Fellman BM, Urbauer DL, Mills GB, Zhang W, Broaddus RR. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol. 2017 Jul;30(7):1032-1041. doi: 10.1038/modpathol.2017.15. Epub 2017 Mar 10. PMID: 28281553; PMCID: PMC5493522.
- Chiarelli S, Buriticá C, Litta P, Ciani S, Guarch R, Nogales FF. An immunohistochemical study of morules in endometrioid lesions of the female genital tract: CD10 is a characteristic marker of morular metaplasia. Clin Cancer Res. 2006 Jul 15;12(14 Pt 1):4251-6. doi: 10.1158/1078-0432.CCR-06-0398. PMID: 16857799.
- Houghton O, Connolly LE, McCluggage WG. Morules in endometrioid proliferations of the uterus and ovary consistently express the intestinal transcription factor CDX2. Histopathology. 2008 Aug;53(2):156-65. doi: 10.1111/j.1365-2559.2008.03083.x. PMID: 18752499.
- Patkar R, Mishra S. Extensive morular metaplasia in an endometrial polyp – A rare occurrence - creating a diagnostic dilemma. IP Arch Cytol Histopathology Res. 2023;8(2):126-129.
- Zhu S, He L, Zheng C, Hou Y. Bladder mulberry-like fibroepithelial polyp with calcification and squamous cell metaplasia mimicking bladder carcinoma: case report and literature review. J Int Med Res. 2020 Jan;48(1):300060519896911. doi: 10.1177/0300060519896911. PMID: 32008408; PMCID: PMC7113808.