More Information
Submitted: February 14, 2024 | Approved: April 24, 2024 | Published: April 25, 2024
How to cite this article: Gupta PD. Sexual Dimorphism in Autoimmune Disorders. Clin J Obstet Gynecol. 2024; 7: 056-058.
DOI: 10.29328/journal.cjog.1001164
Copyright License: © 2024 Gupta PD. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Immune system; Cytokines; Sex hormones; Xist-ribonucleoprotein
Sexual Dimorphism in Autoimmune Disorders
PD Gupta*
Former Director Grade Scientist, Centre for Cellular and Molecular Biology, Hyderabad, India
*Address for Correspondence: PD Gupta, Former Director Grade Scientist, Centre for Cellular and Molecular Biology, Hyderabad, India, Email: [email protected]
Sexual dimorphism exists in Homo sapiens in many systems. Lately, it was found that it also exists in autoimmune disorders. Generally, it was known that the two genders in humans have different endocrine systems, and therefore hormone hormone-regulated systems show sexual dimorphism. However, in the case of autoimmune disorders, it is not due to directly on hormonal milieu but depends on X-chromosome inactivation in males. Whereas every cell in a woman’s body produces Xist; this ribonucleoprotein contains about 81 proteins. This chromosomal inactivation in males and formation of Xist ribonucloprotein in females is responsible for sexual dimorphism in autoimmune disorders in humans.
Men and women two genders belong to the same species but show sexual dimorphism that includes differences in stature, weight, the morphology of the face, cognitive development, mortality, and more importantly disease frequency [1-3]. Autoimmune diseases disproportionately affect females more than males and this is the most prevalent category of disease after cancer and cardiovascular diseases, autoimmune disorders are four-fold higher in females than males [4]. By now, more than 80 autoimmune diseases have been identified by scientists among them, some are well known, for example, type 1 diabetes, multiple sclerosis, lupus, and rheumatoid arthritis, while others are rare and difficult to diagnose [5]. With rare autoimmune disorders, patients may suffer for years before getting a proper diagnosis and treatment. Most autoimmune disorders have no proper cure only symptomatic medicines are provided; some require lifelong treatment to ease symptoms. These diseases are likely the result of genetic and environmental factors interactions. The other causative factors may be the gender, race, and ethnicity [5]. Autoimmune diseases are more common when people are in contact with certain environmental exposures. Indeed, are supposed to be associated with most autoimmune diseases. It was recorded that women have higher absolute levels of antibodies than men and since (auto) antibodies in women are also found at higher levels and therefore, differences in antibodies may cause an increased prevalence of autoimmune disease in women.
Sex differences and the immune system
The milieu of sex hormones differs in men and women, and these hormones have different modes of action in cytokine production, B cell maturation, homing of lymphocytes, and antigen presentation [6,7] in men and women. It was observed that females have more susceptibility, which may be due to estrogen action [7,8]. The human immune system's main functions are antibody production and regulating cellular responses; these two important functions also manifest some degree of sexual dimorphism. Sex-related differences in autoimmune diseases are well documented and showed that females show a greater tendency to develop autoimmune diseases than their male counterparts. Sex hormones, namely dihydrotestosterone and estrogens, have been shown to regulate the severity of inflammatory diseases [7]. In general terms, women have an enhanced antibody production and increased cell-mediated responses following immunization [9] while men produce a more intense inflammatory response to infectious organisms. Further, women have higher CD4+ T cell counts than men which contributes to increased CD4/CD8 ratio [9] and higher levels of plasma IgM production. Sex hormones play an important role in B cell development and function in physiology [8,10,11] and contribute to their dysfunction in autoimmune diseases. It has been known for a long time that estrogen enhances humoral responses, B cell differentiation, and immunoglobulin (Ig) production [7,8,11].
Production of Xist
Mammalian females have a XX and males have a XY genotype. In females (XX) and males (XY), both pairs of chromosomes have roughly equivalent gene expression. But, the X chromosome does not express in males, whereas every cell in a woman’s body produces Xist and not in men.
Chang and team [12] explained how autoimmune diseases in females could be linked with the Xist ribonucleoprotein (RNP) complex. This nucleoprotein comprises a lncRNA, bound RNA binding proteins, and bound to pieces of genomic DNA these autoantigen immune complexes are the hallmark of systemic autoimmune diseases.
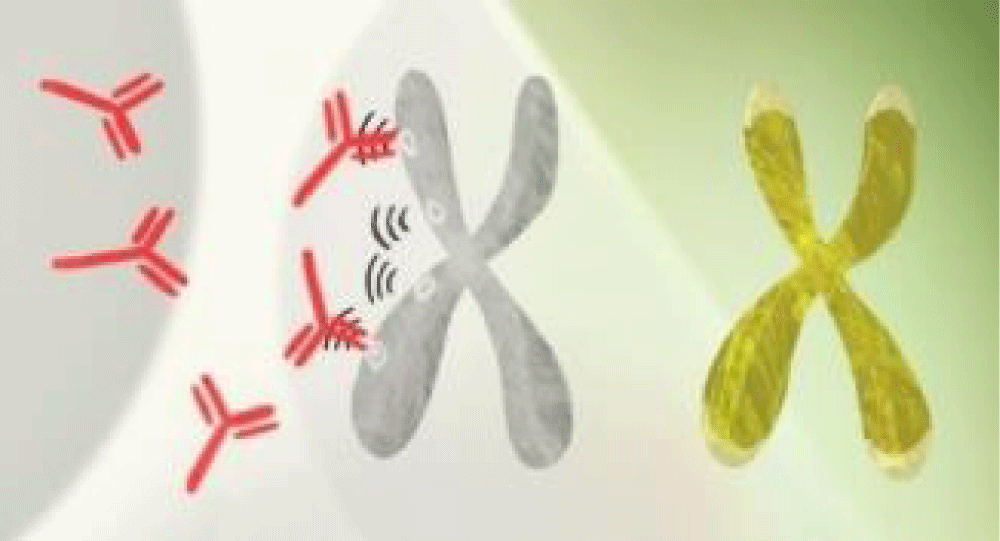
Xist stops one of the two X chromosomes in every cell in the female body as shown in Figure 1. Therefore, due to this imbalance of gene products and altered endogenous material out of normal epigenetic context autoimmune diseases set in more females. This explains the key role of the X chromosome. X chromosome inactivation, a major epigenetic feature in female cells that provides dosage compensation of X-linked genes to avoid over-expression, presents special vulnerabilities that can contribute to the disease process. Xist is critical for the establishment of X chromosome inactivation spreading from the X-inactivation center and coating the entire inactive X in association with its protein partners.
Figure 1: Set of XX chromosomes in females one of them is capable of expressing the Xist ribonucleoprotein (RNP) complex.
Xist associates with 81 unique binding proteins to form a ribonucleo-protein (RNP) complex, through direct RNA protein interaction and others through indirect protein-protein interaction [13-15]. In addition to the female population, males also suffer from autoimmune diseases but do not seem to have the autoimmune condition as badly as females, suggesting there may be other factors at work. Research throws light on the mystery of why women are much more prone to autoimmune disorders: A molecule made by one X chromosome in every female cell can generate antibodies to a woman's tissues.
Researchers noticed that many of the proteins commonly targeted by the immune system in people with autoimmune diseases had something in common: They help a molecule called Xist carry out its function. Xist molecules act a bit like quality control inspectors for women’s extra X chromosomes, preventing them from producing a toxic amount of proteins. The scientists suspect that when immune cells encounter large bunches of these Xist-related proteins—for instance when a dead cell spills them into the bloodstream—they may react by making antibodies to attack them throughout the body. To test the idea, the team studied genetically engineered mice in which both males and females produced Xist. Like their female counterparts, these males were also at an increased risk of developing severe cases of lupus. The researchers also found that people with autoimmune disorders had more antibodies for Xist-related proteins in their blood [16-18]. Still, Xist molecules may not be the only factor at play but multiple research efforts found many more reasons such as: Organic mercury may trigger autoimmune disease – In a study funded by NIEHS, methylmercury, even at exposure levels generally considered safe, may be linked to development of autoimmune antibodies in women of reproductive age. These antibodies could lead in turn to autoimmune diseases, such as inflammatory bowel disease, lupus, rheumatoid arthritis, and multiple sclerosis [19].
Mechanism of antibody generation against Xist-associated proteins (Add these sections after the discussion section)
1. X chromosome inactivation and Xist role: In females, one of the two X chromosomes is inactivated in each cell to ensure dosage compensation between males (XY) and females (XX). This inactivation is mediated by the Xist RNP complex. Xist RNA coats the X chromosome to be inactivated and recruits a variety of proteins and epigenetic modifiers to silence its gene expression.
2. Autoantigen formation: Some proteins that interact with Xist during the XCI process are crucial for its function. These proteins, when bound to Xist RNA, can form complexes that, under certain conditions, might be mistakenly recognized by the immune system as foreign or altered-self components. This misrecognition is especially plausible if these complexes are aberrantly expressed, misfolded, or released into the extracellular space due to cell damage or stress, leading to an autoimmune response.
3. Immune system recognition: The immune system constantly surveys for pathogens and damaged or altered self-cells through pattern recognition receptors and the process of antigen presentation. If proteins associated with the Xist complex are perceived as non-self or altered-self, this can trigger an immune response, including the production of antibodies against these proteins. This response is part of the body's defense mechanism but can become pathological in the context of autoimmune diseases.
Reasons underlying antibody generation against Xist-associated proteins
1. Genetic susceptibility: Individuals may have genetic predispositions that affect how their immune system recognizes self and non-self components. These predispositions can lead to a higher likelihood of developing autoimmune responses against self-antigens, including those associated with Xist.
2. Environmental triggers: Exposure to certain environmental factors, such as viral infections or chemicals, can alter the normal expression or structure of self-proteins, including those associated with Xist. This alteration can make these proteins immunogenic, leading to the development of autoantibodies.
3. Molecular mimicry: There's a possibility that some Xist-associated proteins share epitopes with foreign antigens. The immune system's response to a foreign antigen might inadvertently target self-proteins that resemble the pathogen, leading to an autoimmune response.
4. Breakdown of folerance: Central and peripheral tolerance mechanisms ensure that autoreactive T and B cells are deleted or become unresponsive. However, a breakdown in these tolerance mechanisms can lead to the survival of autoreactive cells that may target Xist-associated proteins.
Gender-specific immune response: Females, having two X chromosomes, might have a higher expression of some X-linked genes before XCI is complete or in cases of XCI escape, leading to an increased antigenic load of X-linked proteins. This increased load could predispose to a higher chance of immune recognition and autoantibody production against Xist-associated proteins.
Autoimmune diseases burden individuals and healthcare systems globally, particularly affecting females. Understanding sexual dimorphism complexity is crucial for tailored prevention, diagnosis, and treatment strategies. Future research should delve into genetic, hormonal, and environmental interactions, exploring gender-specific interventions. Personalized medicine, accounting for sex differences, holds promise for improved outcomes in autoimmune diseases, addressing gender disparities.
- Chamekh M, Casimir G. Editorial: Sexual Dimorphism of the Immune Inflammatory Response in Infectious and Non-infectious Diseases. Front Immunol. 2019; 10:107.
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005 Mar 17;434(7031):400-4. doi: 10.1038/nature03479. PMID: 15772666.
- Chamekh M, Casimir G. Editorial: Sexual Dimorphism of the Immune Inflammatory Response in Infectious and Non-infectious Diseases. Front Immunol. 2019 Feb 5;10:107. doi: 10.3389/fimmu.2019.00107. PMID: 30804936; PMCID: PMC6371857.
- Kronzer VL, Bridges SL Jr, Davis JM 3rd. Why women have more autoimmune diseases than men: An evolutionary perspective. Evol Appl. 2020 Dec 1;14(3):629-633. doi: 10.1111/eva.13167. PMID: 33767739; PMCID: PMC7980266.
- Hodson R. Autoimmune disease. Nature. 2021; 595(Suppl 45):S45.
- Gupta PD. Cytokines: The Game Changer in Pathogenesis of Covid-19. J Clin Ext Immunol. 2020; 5:283.
- Gupta PD. Pathogenesis Due to Inflammation. J Vet Med Ser B. 2018; 8(4):1219.
- Bhatia A, Sekhon HK, Kaur G. Sex hormones and immune dimorphism. ScientificWorldJournal. 2014;2014:159150. doi: 10.1155/2014/159150. Epub 2014 Nov 17. PMID: 25478584; PMCID: PMC4251360.
- Rosenkrands I, Vingsbo-Lundberg C, Bundgaard TJ, Lindenstrøm T, Enouf V, van der Werf S, Andersen P, Agger EM. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine. 2011 Aug 26;29(37):6283-91. doi: 10.1016/j.vaccine.2011.06.040. Epub 2011 Jun 29. PMID: 21722683.
- Gupta PD. Corona Gyan. Capricorn Publishing House; 2020. Jaipur, India.
- Parsa N, Zaheri PM, Hewitt RG, Karimi Akhormeh A, Taravatmanesh S, Wallin L. The rapid CD4 + T-lymphocyte decline and human immunodeficiency virus progression in females compared to males. Sci Rep. 2020 Oct 8;10(1):16816. doi: 10.1038/s41598-020-73852-0. PMID: 33033335; PMCID: PMC7544823.
- Dou DR, Zhao Y, Belk JA, Zhao Y, Casey KM, Chen DC, Li R, Yu B, Srinivasan S, Abe BT, Kraft K, Hellström C, Sjöberg R, Chang S, Feng A, Goldman DW, Shah AA, Petri M, Chung LS, Fiorentino DF, Lundberg EK, Wutz A, Utz PJ, Chang HY. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell. 2024 Feb 1;187(3):733-749.e16. doi: 10.1016/j.cell.2023.12.037. PMID: 38306984; PMCID: PMC10949934.
- McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017 Jan 2;95(1-2):24-39. doi: 10.1002/jnr.23809. PMID: 27870427; PMCID: PMC5120618.
- Lasrado N, Jia T, Massilamany C, Franco R, Illes Z, Reddy J. Mechanisms of sex hormones in autoimmunity: focus on EAE. Biol Sex Differ. 2020 Sep 7;11(1):50. doi: 10.1186/s13293-020-00325-4. PMID: 32894183; PMCID: PMC7475723.
- Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018 Oct 4;9:2279. doi: 10.3389/fimmu.2018.02279. PMID: 30337927; PMCID: PMC6180207.
- Brooks WH, Renaudineau Y. Epigenetics and autoimmune diseases: the X chromosome-nucleolus nexus. Front Genet. 2015 Feb 16;6:22. doi: 10.3389/fgene.2015.00022. PMID: 25763008; PMCID: PMC4329817.
- Lu Z, Guo JK, Wei Y, Dou DR, Zarnegar B, Ma Q, Li R, Zhao Y, Liu F, Choudhry H, Khavari PA, Chang HY. Structural modularity of the XIST ribonucleoprotein complex. Nat Commun. 2020 Dec 2;11(1):6163. doi: 10.1038/s41467-020-20040-3. PMID: 33268787; PMCID: PMC7710737.
- Loda A, Heard E. Xist RNA in action: Past, present, and future. PLoS Genet. 2019 Sep 19;15(9):e1008333. doi: 10.1371/journal.pgen.1008333. PMID: 31537017; PMCID: PMC6752956.
- Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol. 2010 Feb;125(2 Suppl 2):S238-47. doi: 10.1016/j.jaci.2009.09.041. Epub 2010 Jan 12. PMID: 20061009; PMCID: PMC2832720.