Research Article
The predictive value of the preoperative diagnostic tests in mature cystic teratomas of the ovary

Hanife Saglam1, Funda Atalay1*, Ayse Filiz Avsar2 and Hüseyin Levent Keskin3
1University of Health Sciences, Dr. AY Ankara Oncology Education and Research Hospital, Department of Gynecologic Oncology, Ankara, Turkey
2Ankara Yıldırım Beyazıt University, School of Medicine, Department of Obstetrics and Gynecology, Ankara, Turkey
3University of Health Sciences, Zekai Tahir Burak Women’s Health Education and Research Hospital, Department of Obstetrics and Gynecology, Ankara, Turkey
*Address for Correspondence: Funda Atalay, University of Health Sciences, Dr. AY Ankara Oncology Education and Research Hospital, Department of Gynecologic Oncology, Ankara, Turkey, Tel: 905325643555; Email: [email protected]
Dates: Submitted: 06 December 2018; Approved: 18 December 2018; Published: 19 December 2018
How to cite this article: Saglam H, Atalay F, Avsar AF, Keskin HL. The predictive value of the preoperative diagnostic tests in mature cystic teratomas of the ovary. Clin J Obstet Gynecol. 2018; 1: 073-081. DOI: 10.29328/journal.cjog.1001013
Copyright License: © 2018 Saglam H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Mature cystic teratoma; Ovary; Tumor markers: Radiologic imaging
Abstract
Aim: The aim of this study was to determine the sensitivity of the tumor markers and diagnostic methods used in the preoperative period for dermoid cysts, the most common benign neoplasm of the ovary.
Material and Methods: 136 patients who were operated for any reason and reported as ovarian dermoid cyst in the Department of Obstetrics and Gynecology, Ankara Atatürk Training and Research Hospital between January 2004 and September 2005 were included in the study. The medical records of the cases were obtained retrospectively from Ankara-Atatürk Training and Research Hospital, HIS, archive files and patient numbers where necessary.
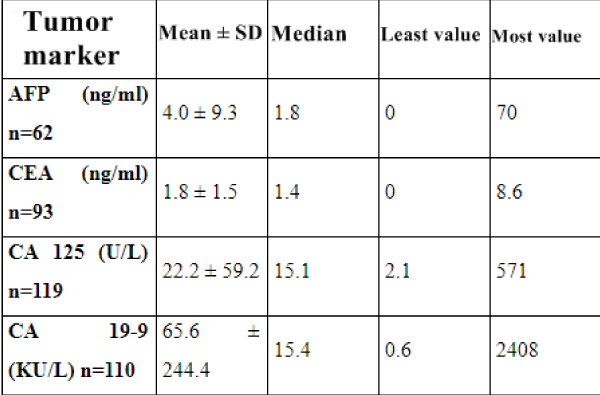
Results: In the preoperative period, 119 patients underwent ultrasonographic examination, 33 underwent Computed Tomography, and 17 underwent Magnetic Resonance Imaging.10 of the cases only underwent CT, while 3 of the cases underwent only MRI 22 of them underwent both USG and CT, USG and MRI were performed on 13 cases and only 1 case underwent all three of the imaging methods. Tumor markers were CEA, CA 125, CA 19-9, CA 15-3 and AFP.
Conclusions: The reviews of ultrasonography and / or computed tomography and / or magnetic resonance imaging (n = 132) revealed that 103 of the cases were put into operation and the sensitivity of the preoperative screening methods were calculated to be 75.5%. The sensitivity of the tumor marker CA 19-9 was calculated to be 31%.
Introduction
Mature cystic teratomas (MCT) constitute 10-25% of all ovarian neoplasia and 60% of all benign ovarian neoplasms [1-3]. MCT forms more than 95% percent of all over teratomas and is always benign [4]. Teratomas consist of a single germ cell and therefore may contain any or all of the three germ layers (ectoderm, mesoderm or endoderm) [5]. These layers typically form tissues with different and irregular structures in the ovary [5,6]. More than 95% of all ovarian teratomas are formed by MCT and is always benign [4]. It is the most common ovarian germ cell tumor in reproductive age women [7]. Approximately 8-17% of the cases were bilateral [1,3,8-10]. Malign transformation can be seen in 0.5.% to 8% of the cases (especially in postmenopausal women). Since these cysts are mostly covered with squamous epithelium, 80% of malignant cases are composed of squamous cell carcinoma [11].
The characteristic macroscopic appearance of MCT is the multicystic mass with hair, teeth, and / or sebaceous / thick, sticky, and usually mixed with malodorous material. There is a solid protrusion (Rokitansky nodule) between the teratoma and normal ovarian tissue [12,13]. MCT may microscopically contain endodermal, mesodermal and ectodermal mature tissues, but generally ectodermal elements outbalance the others [6,14].
These neoplasms, which are always benign, are usually asymptomatic, but may present with abdominal distension, abdominal mass, constipation, nausea, vomiting, or signs of infection, depending on the size of the masses. The most important complications that may develop from MCT are rupture, torsion and malignancy risk. Therefore, surgical removal is recommended [15-17]. Surgical excision provides definitive diagnosis in most women with mature cystic teratomas, eliminating symptoms and preventing complications such as torsion, rupture, and malignant degeneration. As with other ovarian cysts, surgery may be by laparoscopic approach or laparotomy, and cystectomy or oophorectomy may be performed [18].
The basic imaging tool for preoperative examination of MCT is ultrasonography (USG). The density of these tumors varies from full cyst to full solitary and, in particular, unlike many other ovarian tumors of the MCT, these tumors has many sonographic characteristic features such as oil-liquid or hair-liquid levels, bristle density, Rokitansky overhang, Iceberg tip, dot-dash, floating balls sign [6,19,20]. The superiority of magnetic resonance ımaging (MRI) to other imaging modalities used in pelvis include direct multiplanar examination ability, high soft tissue contrast, the ability to differentiate the veins without using contrast media and the safe use of them in pregnant women. Characteristic/typical MRI finding is the fatty tissue seen in about 95% of cases. Other findings are liquid-liquid levels, low signal intensity calcification (usually tooth tissue) and Rokitansky nodules, chemical transition artifact, oil-liquid level, palm tree-like protrusion, floating ball sign, intratumoral keratinoid material [21-25]. Standard T1 and T2 imaging sequences support the diagnosis of teratoma, but T1-weighted out-of-phase or chemically selective fatty tissue imprinted images increase diagnostic confidence. Diagnostic accuracy of these techniques has been reported up to 96%. [22]. Out-of-phase imaging may be particularly advantageous for lesions containing small amounts of fat [23]. Computed tomography (CT) has excellent sensitivity (93-98%) due to the determination of fat in the diagnosis of MCT [23]. Computed tomography includes a mass in fat density with or without fat-fluid level. The high density Rokitansky node consisting of hair and other components can swim in the middle of the mass and calcification is frequently present [26]. The sign of floating balls is a striking and rare finding of MCT and consists of several small spherical structures within the cyst [27].
Tumor markers may show a slight increase in MCT [28]. CA 125, CA 19-9, carcinoembryonic antigen (CEA) are markers that can increase in MCT [29,30].
The aim of this study is to determine the predictive values of the tests performed for diagnosis in the preoperative period and to reach a fast and accurate diagnosis with low cost of this disease, which is frequently encountered in the reproductive period and which has various complications including loss of fertility if not treated.
Material and Method
Between the dates of January 2004 and September 2015, in Ankara Atatürk Training and Research Hospital, Department of Obstetrics and Gynecology a total of 136 patients were included who got operated for whatever reason and reported as ovary mature cystic teratoma. The medical records of the patients were retrospectively reviewed and the patient information (archive files and patient phone numbers) was obtained from hospital recording system. Where necessary. The records of the patients were fully screened; age, gravida, parity, menopause, preoperative imaging methods and histopathological diagnosis were recorded.
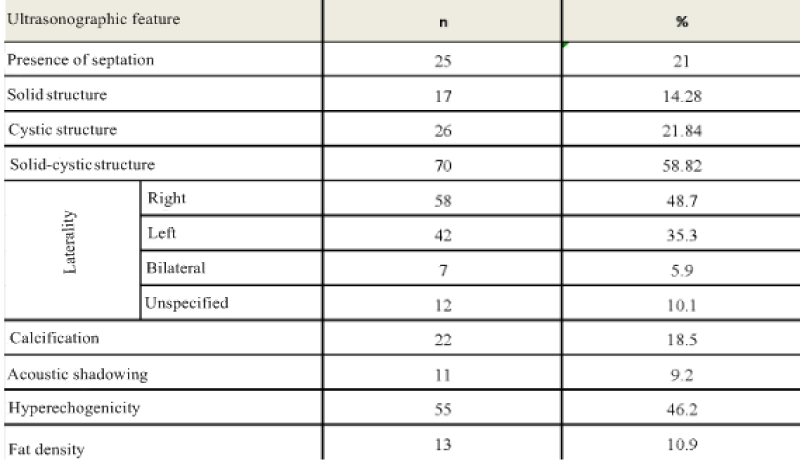
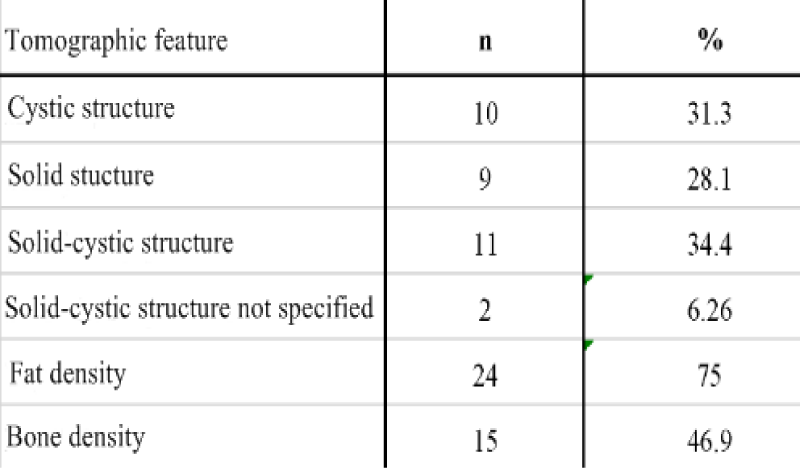
In the preoperative period, 119 patients were diagnosed with USG, 33 patients with CT, and 17 patients with MRI. Only CT was performed on 10 cases, on 3 cases only MRI was performed , CT was performed on 22 patients with USG, MRI was performed with USG on 13 patients, and all three imaging methods were performed on one patient.CT and MRI were done as additional radiological examination when 33 (24.3%) of the patients couldn’t be pre-diagnosed with USG or their tumor markers were high (especially CA-125) or when there was suspicion of malignancy or the result of USG was no good as a result of obesity. The tumor markers AFP, CA-125, CA 15-3, CA 19-9 and CEA were examined. In the USG, the diameter of the tumor, the side of the tumor and whether it is unilateral or bilateral, the presence of septation without consideration of septa thickness, the presence of solid-cystic separation, fat density, presence of acoustic shadowing, calcification and hyper-echogenicity were recorded. The diameter of the tumor, the presence of fat-bone density and the possibility of ‘’dermoid’’ prediction evaluated by computed tomography. The diameter of the tumor, the presence of bone density, and the possibility of the ‘’dermoid’’ were analyzed in the cases that underwent MRI.
Cases who did not pre-diagnosed with ovarian cyst were recorded as incidental. Patient data were analyzed in SPSS 17.0 Statistical Program. Descriptive data were shown as number (n),% (%), mean ± standard deviation (SD), minimum and maximum. Mean values of groups and comparison between groups were done by One-way ANOVA method while evaluating the tumor diameters.
Results
The mean age of the cases was 38.8 ± 13.2 (18-75). The number of cases under 20 years of age was 8 (5.9%). Of the 119 cases who underwent ultrasonography, 80 were diagnosed as MCT preoperative sensitivity of USG was calculated as 67.2%. In CT 26 (78.8%) of the patients were diagnosed as ‘’ MCT’’ while 3 (9.4%) cases were defined as cystic lesions and 2 (6.3%) cases were interpreted as necrotic masses. One (3.1%) patient diagnosed as malignant 1 (3.1%) before surgery, was sigmoid colon mass. 3 (17.7%) of the cases evaluated with MRI showed bone density. While 12 (70.6%) of the cases were diagnosed as MCT, 5 (29.4%) cases were defined as cystic structure. As a result of USG and / or CT and / or MRI (n = 132), 103 patients were operated with the pre-diagnosis of MCT. Preoperative evaluation sensitivity of the imaging methods was calculated to be 75.7%. 26 of the 33 patients evaluated with CT had a preoperative diagnosis of MCT and sensitivity was 78.8%. 12 of the 17 patients with MRI imaging supported the preoperative diagnosis of MCT, with a sensitivity of 70.6%. Sensitivity of tumor markers CA 125, CA 15-3 and AFP were respectively calculated to be 16%, 10.7% and 6% while CA 19-9 and CEA were 31% and 20.4%
Of the 136 patients diagnosed with MCT as a result of histopathological evaluation, 103 had the correct diagnosis of MCT preoperatively, two of which were associated with pregnancy. In 20 cases, the pre-diagnosis was defined as ovarian cyst / adnexial mass and 3 cases presented with acute abdomen and operated with pre-diagnosis of ovarian torsion.In 2 cases, the operation was performed with the preliminary diagnosis of tubaovaryan abscess and ovarian malignancy, but MCT was found in the postoperative diagnosis.In the remaining 8 cases, the indications for surgery were caesarean section (n = 3), acute abdomen (appendectomy, n = 1, ectopic pregnancy, n = 2), endometrial cancer (n = 1), hysterectomy due to myoma (n = 1) and preoperative period. While MCT was not detected by imaging methods, it was reported as mature cystic teratoma as a result of pathological examination of ovarian cyst which was detected intraoperatively.
Histopathological examination results of one patient (0.7%) were reported as immature teratoma, one case (0.7%) as malignant transformation and 2 (1.5%) cases as struma ovarii. Tumor markers CA 19-9 and AFP levels were found in the cases reported above normal values (CA 19-9 = 114 kU/L, AFP= 70.08 ng/ml) in immature teratoma.
In our study, 5 out of 7 cases who were only examined with USG and were reported as bilateral had been diagnosed with MCT in bilateral ovaries while 2 out of 7 didn’t show any signs MCT or any other ovarian pathology. However, 3 of the cases with unilateral ovarian cysts had bilateral MCT in the ovaries tables 1-4.
Discussion
Although these neoplasms, which are frequently seen in the reproductive period, are usually asymptomatic, it is important to diagnose and treat at an early stage because they have various complications, including loss of fertility, if not treated. In the study conducted by Genç et al., 33 patients were examined and 21 patients (63.6%) were correctly diagnosed as MCT by preoperative ultrasonography. These results are similar to our study [31]. Mais et al. determined that sensitivity and specificity were 58% and 99%, respectively according to findings in USG such as, dens echogenic nodules, focal or diffuse echogenic foci, and multiple echogenic lines formed by hairs in MCT [32].
In our study, the ultrasonographic examination of the lesions, solid, cystic, solid-cystic, fat density, calcification, acoustic shadowing, and hyper-echogenicity patterns, preoperative mature cystic teratoma was diagnosed in 80 of 119 cases. Sensitivity of USG was found to be 67.2%. de Kroon et al. found sensitivity to 80% and specificity to 89% in studies involving 99 dermoid cysts [33].
In a study conducted by Tekin et al., of 40 patients with MCT diagnosed as ultrasonographic preliminary diagnoses from 245 patients who were operated with the diagnosis of adnexal mass, the compatibility between the initial diagnosis and the true diagnosis was found to be statistically significant. The sensitivity of ultrasonography (81.8%), specificity (93.1%), PPV (67.5%) and NPV (96.7%) were also found to be high [34]. In the published studies it is stated that acoustic shadowing, focal or diffuse hyperechoic foci, hyperechogenic ridge formed by hair can be seen at the rates of 90%, 60% and 60% again respectively [24]. Tekin et al. reported in their study that even though hyperechoic solid component was detected in 89,5% cases and mixed echogenicity due to hyperechogenic fine lines in 77,4% cases, acoustic shadowing was only detected in 12,5% of the cases [34]. In the same study, it was found out that 13 of 40 patients who were thought to have MCT ultrasonographically, were misdiagnosed and it was showened that the reason of diagnosis of misdiagnosis is to take the criteria of hyperechogenic lining and hyperechogenic solid structure in the pattern recognition method as a diagnostic criteria [34].
Computed tomography has a high sensitivity for detecting MCT. Measurement of fat density is difficult in cases where the fat in the lesion is low. Other than the fat density, palm tree-like protrusion and fat-liquid levels are other specific findings that can be seen on CT. On CT, the fat-liquid level can be seen at 10% [23]. In the study of Saba et al., CT revealed typical features of MCT to be, fat density 93% teeth and calcifications 56% Rokitansky nodule and 81% hair density 65% fat-liquid levels 12% respectively [35-42]. Guerriero et al. evaluated 83 adnexal masses who persisted after 3 months of the 161 cases they examined. They were able to diagnose MCT with 100% sensitivity and specificity in 14 cysts and obtained higher sensitivity when compared with MRI [43]. Similarly, Buy et al. compared the preoperative CT and MRI findings of lesions in 25 cases with histopathological results reported as MCT Rokitansky protrusion, fat and calcified tissue characterization were evaluated in CT and MRI and they found the sensitivity of CT to be 98% and MRI was 88% [44]. In our study, considering the features reported with CT, while the cystic structure was evaluated as 31.3%, solid structure as 28.1%, solid-cystic structure as 34.4%, fat density as 75% and bone density as 46.9%; bone density was determined in 3 (17.7%) of the cases evaluated by MRI and 12 (70.6%) of the cases were diagnosed as “ MCT” without any descriptive characteristic and 5 (29.4%) cases were defined only as cystic structure. Saba et al. in their review of the MRI, fat, fat-liquid, hair densities, palm tree protrusions and protrusions in the appearance of MCT are emphasized as typical features [15-17,45-49].
When we look at the tumor markers in our study, CA 125 sensitivity, which is essentially important in the diagnosis of epithelial ovarian neoplasms, was found to be 16% in the diagnosis of MCT, a germ cell ovarian tumor. Kawai, Kikkawa, and Mikuni have shown that CA-125 values in patients with MCTs have increased by 23.7%, 28% and 12.7%, respectively, which are similar to our data [50-52].
In our study, the sensitivity of CA 15-3 in the diagnosis of MCTwas found to be 10.7%. AFP sensitivity was calculated as 6%. In a study by Konishi et al., CEA levels increased to 30% of cases diagnosed with MCT. Whereas Kawai et al., couldn’t find any significant rise in CEA levels with cases who have MCT [51-53]. In our study, CEA sensitivity was calculated as 20.4%. In a study of Var et al. on 160 patients, CA 19-9 was found in 37.6% of the cases, CA 125 was present in 19.3%, CEA in 9.4%, CA 15-3 in 4% and AFP levels were high in 0,9% of the aforementioned cases. In the study where they detected that CA 19-9 is a more accurate marker in ovarian MCT, they discovered that the most important determinant of CA 19-9 level was the diameter of tumor [54]. In our study, CA 19-9 was higher in MCT than the other tumor markers and in 34 of the 110 cases, it was found to be higher than normal values and its sensitivity was calculated as 31%.
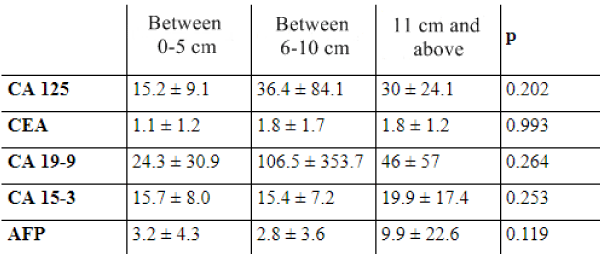
Studies have focused on the correlation of tumor diameter and height of tumor markers. Mikuni and Dede did not find a significant relationship between CA 19-9 level and tumor diameter, but they detected a significant relationship between CA19-9 level and tumor diameter.In the study of Var et al. while it is observed normally in cases with tumors whose diameter is below 4 cm, CA 19-9 levels start to rise in tumors with diameter between 4-10 cm and in tumors which have diameters over 10 cm CA 19-9 levels are still on the rise as well as CA 125 and CEA [52,54,55].
We did not find a relationship between tumor size and height of tumor markers.
Conclusion
MCT is the most common germ cell tumor of the ovary, which constitutes approximately 10-25% of all ovarian neoplasms and 60% of all benign ovarian neoplasms. Although MCT is usually asymptomatic, symptoms in symptomatic cases may present us with symptoms such as abdominal distension, abdominal mass, constipation, nausea, vomiting, infection, torsion or rupture, depending on the size of the masses. The fact that the Rokitansky bulge was not reported at all in our CT and MRI reports and that the characteristics of the mass were not specified were the limitations of our study within the framework of standardization. One of the limitations of our study was that when the imaging methods were reported, the definitions were in the form of general expressions. Although it is called ‘’MCT’’ by the radiologist, it is necessary to have radiologists working on this subject and the procedure should be done by the same radiologist and this situation has not been achieved in our study.
As a result, sonography is the primary imaging tool used to determine MCT. It is seen that we need more descriptive radiology reports to achieve correct pre-operative diagnosis and standardization by supporting with CT and / or MRI. Preoperative tumor markers appear to have no place in the diagnosis and validation of MCT. The thesis that tumor markers increase with increasing tumor size needs to be supported by studies.
References
- Katsube Y, Berg JW, Silverberg SG. Epidemiologic pathology of ovarian tumors: a histopathologic review of primary ovarian neoplasms diagnosed in the Denver Standard Metropolitan Statistical Area, 1 July-31 December 1969 and 1 July-31 December 1979. Int J Gynecol Pathol. 1982; 1: 3-16. Ref.: https://goo.gl/9VS41X
- Koonings PP, Campbell K, Mishell DR Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989; 74: 921-926. Ref.: https://goo.gl/FySxpo
- Peterson WF, Prevost EC, Edmunds FT, Hundley JM Jr, Morris FK. Benign cystic teratomas of the ovary; a clinico-statistical study of 1,007 cases with a review of the literature. Am J Obstet Gynecol. 1955; 70: 368-382. Ref.: https://goo.gl/d9MJpd
- Ayhan A, Bukulmez O, Genc C, Karamursel BS, Ayhan A. Mature cystic teratomas of the ovary: case series from one institution over 34 years. Eur J Obstet Gynecol Reprod Biol. 2000; 88: 153-157. Ref.: https://goo.gl/vF4rN8
- Pantoja E, Rodriguez-Ibanez I, Axtmayer RW, Noy MA, Pelegrina I. Complications of dermoid tumors of the ovary. Obstet Gynecol. 1975; 45: 89-94. Ref.: https://goo.gl/W4iN2k
- Hoffman BL. Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, et al. eds. Wiiliams Gynecology. Texas: Mc Graw Hill. 2012; 267. Ref.: https://goo.gl/M4dgvf
- Yoshioka T, Tanaka T. Immunohistochemical and molecular studies on malignant transformation in mature cystic teratoma of the ovary. J Obstet Gynaecol Res. 1998; 24: 83–90. Ref.: https://goo.gl/AQJXtT
- Caruso PA, Marsh MR, Minkowitz S, Karten G. An intense clinicopathologic study of 305 teratomas of the ovary. Cancer. 1971; 27: 343-348. Ref.: https://goo.gl/TY4E6A
- Ozgur T, Atik E, Silfeler DB, Toprak S. Mature cystic teratomas in our serieswith review of the literature and retrospective analysis. Arch Gynecol Obstet. 2012; 285: 1099-1101. Ref.: https://goo.gl/9sVnp1
- Bagolan P, Giorlandino C, Nahom A, Bilancioni E, Trucchi A, et al. The management of fetal ovarian cysts. J Pediatr Surg. 2002; 37: 25-30. Ref.: https://goo.gl/GhynQd
- Chiang AJ, La V, Peng J, Yu KJ, Teng NN. Squamous cell carcinoma arising from mature cystic teratoma of the ovary. Int J Gynecol Cancer. 2011; 21: 466-474. Ref.: https://goo.gl/hbohPb
- Comerci JT Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994; 84: 22-28. Ref.: https://goo.gl/eDC874
- Breen JL, Maxson WS. Ovarian tumors in children and adolescents. Clin Obstet Gynecol. 1977; 20: 607-623. Ref.: https://goo.gl/Jie1x1
- Giorlandino C1, Bilancioni E, Bagolan P, Muzii L, Rivosecchi M, et al. Antenatal ultrasonographic diagnosis and management of fetal ovarian cysts. Int J Gynaecol Obstet. 1994; 44: 27-31. Ref.: https://goo.gl/ejMv5T
- Salman W, Singh M, Twaij Z. A case of papillary thyroid carcinoma in struma ovarii and review of the literature. Patholog Res Int. 2010; 2010: 352476. https://goo.gl/UJHLSx
- Lal S, Singh A, Goel N. Acute gynaecological emergency caused by ruptured dermoid. JEMDS. 2014; 3: 5705-5710. Ref.: https://goo.gl/fEmrba
- Artunc UB, Goker A, Pala HG, Ordu S. Abnormal elevated ca 19-9 in the dermoid cyst: a sign of the ovarian torsion? Case Rep Obstet Gynecol. 2013; 2013: 860505 Ref.: https://goo.gl/JnNTxM
- Comerci JT Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994; 84: 22-28. Ref.: https://goo.gl/6SCsPM
- Sahin H, Abdullazade S2, Sanci M. Mature cystic teratoma of the ovary: a cutting edge overview on imaging features. Insights Imaging. 2017: 8: 227-241. Ref.: https://goo.gl/LUn7ff
- Patel MD, Feldstein VA, Lipson SD, Chen DC, Filly RA. Cystic teratoma of the ovary: diagnostic value of sonography. AJR Am J Roentgenol. 1998; 171: 1061–1065 Ref.: https://goo.gl/C1Ki8w
- Dooms GC, Hricak H, Tscholakoff D. Adnexal structures: MR imaging. Radiology. 1986; 158: 639-646. Ref.: https://goo.gl/Ax2kXj
- Mitchell DG, Mintz MC, Spritzer CE, Gussman D, Arger PH, et al. Adnexal masses: MR imaging observations at 1.5 T, with US and CT correlation. Radiology. 1987; 162: 319-324. Ref.: https://goo.gl/RFYAxm
- Saba L, Guerriero S, Sulcis R, Virgilio B, Melis G, et al. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur J Radiol. 2009; 72: 454–463. Ref.: https://goo.gl/tp3VZG
- Togashi K, Nishimura K, Itoh K, FujisawaI, Sago T, et al. Ovarian cystic teratomas: MR imaging. Radiology. 1987; 162: 669–673. Ref.: https://goo.gl/kgcB16
- Nakamaya T, Yoshimitsu K, Irie H, Aibe H, Tajima T, et al. Diffusion- weighted echo-planar MR imaging and ADC mapping in the differential diagnosis of ovarian cystic masses: usefulness of detecting keratinoid substances in mature cystic teratomas. J Magn Reson Imaging. 2005; 22: 271–278. Ref.: https://goo.gl/CWRi7M
- Matsumoto F, Yoshioka H, Hamada T, Ishida O, Noda K. Struma ovarii: CT and MR findings. J Comput Assit Tomgr. 1990; Mr-Opr; 14: 310-312. Ref.: https://goo.gl/CDPsU4
- Mahomedy S, Bayat MR, Seedat M. Meat balls: a pathognomonic ultrasound and computed tomography finding in mature cystic teratoma. Australas Radiol. 2007; 51: B281–B283 Ref.: https://goo.gl/EXCEFB
- Hackethal A, Brueggmann D, Bohlmann MK. Et al. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008; 9: 1173-1780. Ref.: https://goo.gl/bhdJNk
- Yüksel MA, Abal› R, Aras Ö, Temel , Ünal F, Boran AB, Purisa S. Ovaryan Dermoid Kistlerde CA 19-9 Seviyesinin De¤erlendirilmesi. Türkiye Klinikleri Jinekoloji-Obstetrik Dergisi. 2011; 21: 190-194. Ref.: https://goo.gl/CAGWZy
- Günay E, Kalender HS, Cengizo¤lu B, Turan C, Ünal O. Adneksiyal Kitlelerde Tümör ‘’Marker’’lar› ve Cerrahi Sonuçlar›. Kartal e¤itim Arafl. Hast. T›p Dergisi. 2002; 3: 179-181 Ref.: https://goo.gl/cWTAJB
- Genç M, Şahin N, Karaarslan S. Mature Cystic Teratoma of the Ovary Clinical, Radiological and, Histopathologic Aspects. Journal of Clinical and Analytical Medicine. 2016; 370-374. Ref.: https://goo.gl/nCnCb8
- Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, et al. Transvaginal ultrasonography in the diagnosis of cystic teratoma. Obstet Gynecol. 1995; 85: 48-52. Ref.: https://goo.gl/eYdBTg
- de Kroon CD, van der Sandt HA, van Houwelingen JC, Jansen FW. Sonographic assesment of non-malignant ovarian cysts; does sonohistology exist? Hum Reprod. 2004; 19: 2138-2143. Ref.: https://goo.gl/nviKxF
- Tekin YB, Altınbaş ŞK, Dede S, et al. The parameters affecting accuracy of ultrasonographic diagnosis in dermoid cysts. Dicle Medical Journal. 2014; 41: 64-70.
- Andolf E, Jorgensen C. A prospective comparison of transabdominal and transvaginal ultrasound with surgical findings in gynecologic disease. J Ultrasound Med. 1990; 9: 71–75. Ref.: https://goo.gl/6U152N
- Hata K, Hata T, Manabe A, Sugimura K, Kitao M. A critical evaluation of transvaginal Doppler studies, transvaginal sonography, magnetic resonance imaging, and CA 125 in detecting ovarian cancer. Obstet Gynecol. 1992; 80: 922–926. Ref.: https://goo.gl/V96WPQ
- Kurjak A, Predanic M, Kupesic-Urek S, Jukic S. Transvaginal color and pulsed Doppler assessment of adnexal tumor vascularity. Gynecol Oncol. 1993; 50: 3-9. Ref.: https://goo.gl/JzBDVz
- Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994; 170: 81-85. Ref.: https://goo.gl/YXHJw3
- Quinn SF, Erickson S, Black WC. Cystic ovarian teratomas: the sonographic appearance of the dermoid plug. Radiology. 1985; 155: 477–478. Ref.: https://goo.gl/92kK3R
- Dodd GD, Budzik RF. Lipomatous tumors of the pelvis in women: spectrum of imaging findings. AJR Am J Roentgenol. 1990; 155: 317–322. https://goo.gl/QThLmR
- Sheth S, Fishman EK, Buck JL, Hamper UM, Sanders RC. The variable sonographic appearances of ovarian teratomas: correlation with CT. AJR Am J Roentgenol. 1988; 151: 331–334. Ref.: https://goo.gl/fTNPZ8
- Fridman AC, Pyatt RS, Hartmann DS, Downey EF, Olson WB. CT of benign cystic teratomas. AJR Am J Roentgenol. 1982; 138: 659–665. Ref.: https://goo.gl/7L8AR8
- Guerriero S, Mallarini G, Ajossa S, Risalvato A, Satta R, et al. Transvaginal ultrasound and computer tomography combined with clinical parameters and CA-125 determinations in the differential diagnosis of persistent ovarian cysts in premenopausal women. Ultrasound Obstet Gynecol. 1997; 9: 339–334 Ref.: https://goo.gl/5MbPtf
- Buy JN, Ghossain MA, Moss AA, Bazot M, Doucet M, et al. Cystic teratoma of the ovary: CT detection. Radiology. 1989; 171: 697–701. Ref.: https://goo.gl/iyRSXy
- Laufer M, Goldstein D. Benign and malignant ovarian masses. In: Emans S; Laufer M Goldstein, D, editors. Pediatric and Adolescent Gynecology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. 706-710.
- Zeyneloğlu HB, Öktem M, Eroğlu D, Kuşçu E. Matür kistik teratomlara laparoskopik yaklaşım: Başkent Üniversitesi deneyimi. J Turk Soc Obstet Gynecol. 2005; 2: 116-120. Ref.: https://goo.gl/kLKVkY
- Leibman AJ, Kruse B, McSweeney MB. Transvaginal sonography: comparison with transabdominal sonography in the diagnosis of pelvic masses. Am J Roentgenol. 1988; 151: 89–92. Ref.: https://goo.gl/FjzmuN
- Guttman PH Jr: In search of the elusive benign cystic ovarian teratoma: application of the ultrasound “tip of the iceberg” sign. J Clin Ultrasound. 1977; 5: 403-406. Ref.: https://goo.gl/VNEVR2
- Bronshtein M, Yoffe N, Brandes JM, Blumenfeld Z. Hair as a sonographic marker of ovarian teratomas: improved identifi cation using transvaginal sonography and simulation model. J Clin Ultrasound. 1991; 19: 351-355. Ref.: https://goo.gl/36yfQk
- Kikkawa F, Nawa A, Tamakoshi K, Ishikava H, Kuzuya K, et al. Diagnosis of squamous cell carcinoma arising from mature cystic teratoma of the ovary. Cancer. 1998; 82: 2249–2255. Ref.: https://goo.gl/6CNZ3A
- Kawai M, Kano T, Kikkawa F, Morikawa Y, Oguchi H, et al. Seven tumor markers in benign and malignant germ cell tumors of the ovary. Gynecol Oncol. 1992; 45: 248–253. Ref.: https://goo.gl/Fj3BcK
- Mikuni M, Makinoda S, Tanaka T, Okuda T, Domon H, et al. Evaluation of tumor markers in ovarian dermoid cyst. Acta Obstet Gynaecol Jpn. 1990; 42: 479–484. Ref.: https://goo.gl/F8y11Z
- Konishi I, Fujii S, Okamura H, Sakahara H, Endo K, et al. Analysis of serum CA125, CEA, AFP, LDH levels and LDH isoenzymes in patients with ovarian tumors—correlation between tumor markers and histological types of ovarian tumors. Acta Obstet Gynaecol Jpn. 1986; 38: 827–836. Ref.: https://goo.gl/hhhYM1
- Var T, Tonguc EA, Ugur M, Altinbas S, Tokmak A. Tumor markesrs panel and tumor size of ovarian dermoid tumors in reproductive age. Bratisl Lek Listy. 2012; 113: 95-98. Ref.: https://goo.gl/cSrtyE
- Dede M, Gungor S, Yenen MC, Alanbay I, Duru NK, et al. CA19-9 may have clinical significance in mature cystic teratomas of the ovary. Int J Gynecol Cancer. 2006; 16: 189-193. Ref.: https://goo.gl/Pv6G7E