Abstract
Review Article
Detrimental Effects of Methylenetetrahydrofolate Reductase (MTHFR) Gene Polymorphism on Human Reproductive Health: A Review
Vandana Rai* and Pradeep Kumar
Published: 05 March, 2025 | Volume 8 - Issue 1 | Pages: 007-014
Methylenetetrahydrofolate Reductase (MTHFR) is an important enzyme of the folate cycle, which is required to convert 5,10-methyltetrahydrofolate into 5-methyltetrahydrofolate (5-methylTHHF). 5-methyl THF is a methyl group donor for several cellular methylation processes. It also donates methyl group for the conversion of homocysteine into methionine, the higher concentration of which is toxic. MTHFR gene C677T polymorphism is clinically important polymorphism and the variant MTHFR (A222V) enzyme has reduced activity, hence increasing the requirement for folic acid. Less conversion of folate to 5-methyl-THF due to C677T polymorphism results in a higher plasma concentration of homocysteine (hyperhomocysteinemia). Individuals having C677T polymorphism are susceptible to various diseases, including reproductive problems like male infertility, polycystic ovary syndrome, Recurrent Pregnancy Loss (RPL), Preeclampsia (PE), placental abruption, and adverse pregnancy outcomes. MTHFR C677T polymorphism mimics folate deficiency, and folate is required for DNA synthesis, repair, methylation, and proper chromosome segregation, and all these processes are important for foetal growth and normal development. Methylation and demethylation processes control the gene expression of about 45% of human genes. Impaired methylation influences the expression of genes involved in the regulation of hormones, spermatogenesis, and oogenesis. In males, oxidative stress damages sperm DNA decreases sperm motility, and may impair fertilization capability. In pregnant women, hyperhomocysteinemia increases oxidative stress and inflammation within the placenta, which causes damage to placental tissue, impairs its function, and disrupts foetal development. Further, hyperhomocysteinemia (HHcy) is embryotoxic and neurotoxic and is responsible for congenital anomalies in the foetus. This review supports the idea that MTHFR C677T polymorphism is associated with an increased risk for male infertility, PCOS, RPL, PE, and congenital anomalies. This review may provide a clue toward a better understanding of the correlation between the MTHFR C677T polymorphism and its detrimental effects on human reproductive health.
Read Full Article HTML DOI: 10.29328/journal.cjog.1001182 Cite this Article Read Full Article PDF
Keywords:
MTHFR; Folate; Homocysteine; Male infertility; Preeclampsia; PCOS; Congenital anomalies
References
- Gos M, Szpecht-Potoka A. Genetic basis of neural tube defects. II. Genes correlated with folate and methionine metabolism. J Appl Genet. 2002;5:511-524. Available from: https://pubmed.ncbi.nlm.nih.gov/12441636/
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111-113. Available from: https://pubmed.ncbi.nlm.nih.gov/7647779/
- Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7-9. Available from: https://doi.org/10.1161/01.cir.93.1.7
- Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet. 1998;62:1258-1260. Available from: https://doi.org/10.1086/301836
- Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M, et al. Geographical and ethnic variation of the 677CT allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas worldwide. J Med Genet. 2003;40:619-625. Available from: https://doi.org/10.1136/jmg.40.8.619
- Spiridonova MG, Stepanov VA, Maksimova NR, Puzyrev VP. Population study of frequency of methylenetetrahydrofolate reductase C677T gene polymorphism in Yakutia. Genetika. 2004;40:704-708. Available from: https://pubmed.ncbi.nlm.nih.gov/15272569/
- Rai V, Yadav U, Kumar P, Yadav SK. Methylenetetrahydrofolate reductase polymorphism (C677T) in Muslim population of Eastern Uttar Pradesh, India. Indian J Med Sci. 2010;64(5):219-223. Available from: https://pubmed.ncbi.nlm.nih.gov/22842321/
- Rai V, Yadav U, Kumar P. Genotype prevalence and allele frequencies of 5, 10-methylenetetrahydrofolate reductase (MTHFR) C677T mutation in two caste groups of India. Cell Mol Biol. 2012;58:OL1695-1701. Available from: https://www.cellmolbiol.org/index.php/CMB/article/view/553/226
- Yadav U, Kumar P, Gupta S, Rai V. Distribution of MTHFR C677T gene polymorphism in healthy North Indian population and an updated meta-analysis. Ind J Clin Biochem. 2018;32(4):399-410. Available from: https://doi.org/10.1007/s12291-016-0619-0
- A ZC, Yang Y, Zhang SZ, Li N, Zhang W. Single nucleotide polymorphism C677T in the methylenetetrahydrofolate reductase gene might be a genetic risk factor for infertility in Chinese men with azoospermia or severe oligozoospermia. Asian J Androl. 2007;9:57-62. Available from: https://doi.org/10.1111/j.1745-7262.2007.00225.x
- Rai V, Yadav U, Kumar P, Yadav SK, Mishra OP. Maternal methylenetetrahydrofolate reductase C677T polymorphism and Down syndrome risk: A meta-analysis from 34 studies. PLoS One. 2014;9(9):e108552. Available from: https://doi.org/10.1371/journal.pone.0108552
- Rai V. Strong association of C677T polymorphism of methylenetetrahydrofolate reductase gene with nonsyndromic cleft lip/palate (nsCL/P). Ind J Clin Biochem. 2017;33(1):5-15. Available from: https://doi.org/10.1007/s12291-017-0673-2
- Yadav U, Kumar P, Yadav SK, Mishra OP, Rai V. Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: An updated meta-analysis. Metab Brain Dis. 2015;30:7-24. Available from: https://doi.org/10.1007/s11011-014-9575-7
- Rai V. The MTHFR C677T polymorphism and hyperuricemia risk: A meta-analysis of 558 cases and 912 controls. Metabolomics. 2016;6:166. Available from: http://dx.doi.org/10.4172/2153-0769.1000166
- Rai V. Evaluation of methylenetetrahydrofolate reductase gene variant (C677T) as risk factor for bipolar disorder. Cell Mol Biol. 2011;57:1558-1566. Available from: https://pubmed.ncbi.nlm.nih.gov/21955385/
- Yadav U, Kumar P, Gupta S, Rai V. Role of MTHFR C677T gene polymorphism in the susceptibility of schizophrenia: An updated meta-analysis. Asian J Psychiatry. 2016;20:41-51. Available from: https://doi.org/10.1016/j.ajp.2016.02.002
- Rai V, Kumar P. Methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to epilepsy. Neurol Sci. 2018;39:2033-2041. Available from: https://doi.org/10.1007/s10072-018-3583-z
- Rai V, Kumar P. Relation between methylenetetrahydrofolate reductase polymorphisms (C677T and A1298C) and migraine susceptibility. Ind J Clin Biochem. 2017;37:3-17. Available from: https://doi.org/10.1007/s12291-021-01000-0
- Rai V. Folate pathway gene MTHFR C677T polymorphism and risk of lung cancer in Asian populations. Asian Pac J Cancer Prev. 2014;15(21):9259-9264. Available from: https://doi.org/10.7314/apjcp.2014.15.21.9259
- Kumar P, Yadav U, Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and breast cancer risk: Evidence for genetic susceptibility. MetaGene. 2015;6:72-84. Available from: https://doi.org/10.1016/j.mgene.2015.08.008
- Kumar P, Rai V. Methylenetetrahydrofolate reductase C677T polymorphism and risk of esophageal cancer: An updated meta-analysis. Egypt J Med Hum Genet. 2018;19(4):273-284. Available from: https://www.ajol.info/index.php/ejhg/article/view/181774
- Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and its association with ovary cancer. J Health Med Inform. 2016;7:3. Available from: https://www.hilarispublisher.com/open-access/methylenetetrahydrofolate-reductase-gene-c677t-polymorphism-and-itsassociation-with-ovary-cancer-2157-7420-1000226.pdf
- Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci. 2015;8:191-196. Available from: https://doi.org/10.4103/0974-1208.170370
- Carrell DT. The sperm epigenome: Implications for assisted reproductive technologies. Adv Exp Med Biol. 2019;1166:47-56. Available from: https://doi.org/10.1007/978-3-030-21664-1_3
- Pei J. Association between MTHFR C677T polymorphism and male infertility in Han population of He Nan, China. China Health Care Nutr. 2013;7:629-630.
- Karimian M, Colagar AH. Association of C677T transition of the human methylenetetrahydrofolate reductase (MTHFR) gene with male infertility. Reprod Fertil Dev. 2016;28(6):785-794. Available from: https://doi.org/10.1071/RD14186
- Ni W, Li H, Wu A, Zhang P, Yang H, Yang X, et al. Lack of association between genetic polymorphisms in three folate-related enzyme genes and male infertility in the Chinese population. J Assist Reprod Genet. 2015;32(3):369-374. Available from: https://link.springer.com/article/10.1007/s10815-014-0423-9
- Rai V, Kumar P. Methylenetetrahydrofolate reductase C677T polymorphism and risk of male infertility in Asian population. Ind J Clin Biochem. 2017;32:253-260. Available from: https://doi.org/10.1007/s12291-017-0640-y
- Singh K, Jaiswal D. One-carbon metabolism, spermatogenesis, and male infertility. Reprod Sci. 2013;20:622-630. Available from: https://doi.org/10.1177/1933719112459232
- Cui K, Luan Y, Tang Z, Li CC, Wang T, Wang SG, et al. Human tissue kallikrein-1 protects against the development of erectile dysfunction in a rat model of hyperhomocysteinemia. Asian J Androl. 2019;21:508-515. Available from: https://doi.org/10.4103/aja.aja_111_18
- Salvio G, Ciarloni A, Cutini M, Balercia G. Hyperhomocysteinemia: Focus on endothelial damage as a cause of erectile dysfunction. Int J Mol Sci. 2021;22:418. Available from: https://doi.org/10.3390/ijms22010418
- De Leonardis F, Colalillo G, Finazzi Agrò E, Fuschi A, Asimakopoulos AD. Endothelial dysfunction, erectile deficit, and cardiovascular disease: An overview of the pathogenetic links. Biomedicines. 2022;10:1848. Available from: https://doi.org/10.3390/biomedicines10081848
- Benedetti S, Tagliamonte MC, Catalani S, Benedetti S, Tagliamonte MC, Catalani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online. 2012;25:300-306. Available from: https://doi.org/10.1016/j.rbmo.2012.05.011
- Atig F, Raffa M, Ali HB, Abdelhamid K, Saad A, Ajina M. Altered antioxidant status and increased lipid per-oxidation in seminal plasma of Tunisian infertile men. Int J Biol Sci. 2012;8:139-149. Available from: https://doi.org/10.7150/ijbs.8.139
- Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods, and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371-389. Available from: https://doi.org/10.1093/humupd/dmx006
- Hosen MB, Islam MR, Begum F, Kabir Y, Howlader MZ. Oxidative stress-induced sperm DNA damage, a possible reason for male infertility. Iran J Reprod Med. 2015;13:525-532. Available from: https://pubmed.ncbi.nlm.nih.gov/26568756/
- Aitken RJ, Drevet JR. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants. 2020;9:111. Available from: https://doi.org/10.3390/antiox9020111
- Rashki Ghaleno L, Alizadeh A, Drevet JR, Shahverdi A, Valojerdi MR. Oxidation of sperm DNA and male infertility. Antioxidants. 2021;10:97. Available from: https://doi.org/10.3390/antiox10010097
- Kaltsas A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina (Kaunas). 2023;59:1769. Available from: https://doi.org/10.3390/medicina59101769
- Jurewicz J, Radwan M, Wielgomas B, Sobala W, Piskunowicz M, Radwan P, Bochenek M, Hanke W. The effect of environmental exposure to pyrethroids and DNA damage in human sperm. Systems Biology in Reproductive Medicine. 2015;61(1):37-43. Available from: https://doi.org/10.3109/19396368.2014.981886
- Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur J Obstet Gynecol Reprod Biol. 2019;8(3):100060. Available from: https://doi.org/10.1016/j.eurox.2019.100060
- Cooper HE, Spellacy WN, Prem KA, Cohen WD. Hereditary factors in the Stein-Leventhal syndrome. Am J Obstet Gynecol. 1968;100(3):371-387. Available from: https://doi.org/10.1016/s0002-9378(15)33704-2
- Glueck CJ, Wang P, Fontaine RN, Sieve-Smith L, Tracy T, Moore SK. Plasminogen activator inhibitor activity: An independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metabolism. 1999;48(12):1589-1595. Available from: https://doi.org/10.1016/s0026-0495(99)90250-0
- Jiao X, Chen W, Zhang J, Wang W, Song J, Chen D, et al. Variant alleles of the ESR1, PPARG, HMGA2, and MTHFR genes are associated with polycystic ovary syndrome risk in a Chinese population: A case-control study. Front Endocrinol. 2018;9:504. Available from: https://doi.org/10.3389/fendo.2018.00504
- Almukhtar AA, Almohaidi AMS. Investigation for variation in MTHFR gene in Iraqi Arab females with PCOS. Asian J Microbiol Biotechnol Environ Sci. 2019;21(4):851-861.
- Rai V, Kumar P. Association between methylenetetrahydrofolate reductase gene C677T polymorphism and susceptibility to polycystic ovary syndrome. Ind J Clin Biochem. Available from: https://doi.org/10.1007/s12291-024-01200-4
- Rai V. Methylenetetrahydrofolate reductase C677T polymorphism and recurrent pregnancy loss risk in Asian population: A meta-analysis. Ind J Clin Biochem. 2016;31(4):402-413. Available from: https://doi.org/10.1007/s12291-016-0554-0
- Ahangari N, Doosti M, Mousavifar N, Attaran M, Shahrokhzadeh S, Memarpour S, et al. Hereditary thrombophilia genetic variants in recurrent pregnancy loss. Arch Gynecol Obstet. 2019;300(3):777-782. Available from: https://doi.org/10.1007/s00404-019-05224-7
- Trifonova EA, Swarovskaya MG, Ganzha OA, Voronkova OV, Gabidulina TV, Stepanov VA. The interaction effect of angiogenesis and endothelial dysfunction-related gene variants increases the susceptibility of recurrent pregnancy loss. J Assist Reprod Genet. 2019;36:717-726. Available from: https://doi.org/10.1007/s10815-019-01403-2
- Zarfeshan FY, Kooshkaki O, Kordi TD, Anani SG. Investigation of the association between C677T polymorphism of the MTHFR gene and plasma homocysteine level in recurrent fetal miscarriage. J Obstet Gynaecol Res. 2019;45:1442-1447. Available from: https://doi.org/10.1111/jog.13989
- Shaker MM, Shalabi TA, Amr KS. Correlation of methylation status in MTHFR promoter region with recurrent pregnancy loss. J Genet Eng Biotechnol. 2021;19:44. Available from: https://doi.org/10.1186/s43141-021-00147-w
- Biswas A, Choudhry P, Mittal A, Meena A, Ranjan R, Choudhry VP, et al. Recurrent abortions in Asian Indians: No role of factor V Leiden Hong Kong/Cambridge mutation and MTHFR polymorphism. Clin Appl Thromb Hemost. 2008;14:102-104. Available from: https://doi.org/10.1177/1076029607303774
- Mishra J, Talwar S, Kaur L, Chandiok K, Yadav S, Puri M, et al. Differential global and MTHFR gene specific methylation patterns in preeclampsia and recurrent miscarriages: A case-control study from North India. Gene. 2019;704:68-73. Available from: https://doi.org/10.1016/j.gene.2019.04.036
- Joksic I, Mikovic Z, Filimonovic D, Munjas J, Karadzov ON, Egic A, et al. Combined presence of coagulation factor XIII V34L and plasminogen activator inhibitor 1 4G/5G gene polymorphisms significantly contribute to recurrent pregnancy loss in Serbian population. J Med Biochem. 2020;39:199-207. Available from: https://doi.org/10.2478/jomb-2019-0028
- Wen Y, He H, Zhao K. Thrombophilic gene polymorphisms and recurrent pregnancy loss: A systematic review and meta-analysis. J Assist Reprod Genet. 2023;40:1533-1558. Available from: https://doi.org/10.1007/s10815-023-02823-x
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Preeclampsia. Lancet. 2010;376:631-644. Available from: https://doi.org/10.1016/s0140-6736(10)60279-6
- Redman CW, Sargent IL. Placental stress and preeclampsia: A revised view. Placenta. 2009;30(Suppl A):S38-S42. Available from: https://doi.org/10.1016/j.placenta.2008.11.021
- Chedraui P, Andrade ME, Salazar-Pousada D, Escobar GS, Hidalgo L, Ramirez C, et al. Polymorphisms of the methylenetetrahydrofolate reductase gene (C677T and A1298C) in the placenta of pregnancies complicated with preeclampsia. Gynecol Endocrinol. 2015;31(7):569-572. Available from: https://doi.org/10.3109/09513590.2015.1031104
- Zhou L, Cheng L, He Y, Gu Y, Wang Y, Wang C. Association of gene polymorphisms of FV, FII, MTHFR, SERPINE1, CTLA4, IL10, and TNFalpha with pre-eclampsia in Chinese women. Inflamm Res. 2016;65(9):717-724. Available from: https://doi.org/10.1007/s00011-016-0953-y
- Jafari A, Parchami S, Reiisi S, Miraj S. Association of plasma homocysteine, folic acid levels, and C677T polymorphism in methylene tetra hydrofolate reductase with risk of preeclampsia: A case-control study in Iranian women. Clin Exp Obstet Gynecol. 2018;45(3):367-374. Available from: https://doi.org/10.12891/ceog4032.2018
- Zhang J, Han L, Li W, Chen Q, Lei J, Long M, Yang W, Li W, Zeng L, Zeng S. Early prediction of preeclampsia and small for-gestational-age via multi-marker model in Chinese pregnancies: A prospective screening study. BMC Pregnancy Childbirth. 2019;19:304. Available from: https://doi.org/10.1186/s12884-019-2455-8
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151-2161. Available from: https://doi.org/10.1016/s0140-6736(12)60560-1
- Monangi NK, Brockway HM, House M, Zhang G, Muglia L. The genetics of preterm birth: Progress and promise. Semin Perinatol. 2015;39:574-583. Available from: https://doi.org/10.1053/j.semperi.2015.09.005
- Stalman SE, Solanky N, Ishida M, Alemán-Charlet C, Abu-Amero S, Alders M, Alvizi L, Baird W, Demetriou C, Henneman P, et al. Genetic analyses in small-for-gestational-age newborns. J Clin Endocrinol Metab. 2018;103:917-925. Available from: https://doi.org/10.1210/jc.2017-01843
- Anil KC, Basel PL, Singh S. Low birth weight and its associated risk factors: Health facility-based case-control study. PLoS ONE. 2020;15:e0234907. Available from: https://doi.org/10.1371/journal.pone.0234907
- Dahman HAB. Risk factors associated with preterm birth: A retrospective study in Mukalla Maternity and Childhood Hospital, Hadhramout Coast, Yemen. Sudan J Paediatr. 2020;20:99-110. Available from: https://doi.org/10.24911/sjp.106-1575722503
- Sukla KK, Tiwari PK, Kumar A, Raman R. Low birthweight (LBW) and neonatal hyperbilirubinemia (NNH) in an Indian cohort: Association of homocysteine, its metabolic pathway genes and micronutrients as risk factors. PLoS ONE. 2013;8:e71587. Available from: https://doi.org/10.1371/journal.pone.0071587
- Wang BJ, Liu MJ, Wang Y, Dai JR, Tao JY, Wang SN, Zhong N, Chen Y. Association between SNPs in genes involved in folate metabolism and preterm birth risk. Genet Mol Res. 2015;14(1):850-859. Available from: https://doi.org/10.4238/2015.february.2.9
- Hwang IW, Kang YD, Kwon BN, Hong JH, Han SH, Kim JS, Park JW, Jin HJ. Genetic variations of MTHFR gene and their association with preterm birth in Korean women. Medicina. 2017;53:380-385. Available from: https://doi.org/10.1016/j.medici.2018.01.001
- Tiwari D, Bose PD, Das S, Das CR, Datta R, Bose S. MTHFR (C677T) polymorphism and PR (PROGINS) mutation as genetic factors for preterm delivery, fetal death and low birth weight: A Northeast Indian population-based study. Meta Gene. 2015;3:31-42. Available from: https://doi.org/10.1016/j.mgene.2014.12.002
- Kramer MS, Goulet L, Lydon J, Séguin L, McNamara H, Dassa C, et al. Socio-economic disparities in preterm birth: Causal pathways and mechanisms. Paediatr Perinat Epidemiol. 2001;15(Suppl. 2):104-123. Available from: https://doi.org/10.1046/j.1365-3016.2001.00012.x
- Nan Y, Li H. MTHFR genetic polymorphism increases the risk of preterm delivery. Int J Clin Exp Pathol. 2015;8:7397-7402. Available from: https://pubmed.ncbi.nlm.nih.gov/26261642/
- Wang BJ, Liu MJ, Wang Y, Dai JR, Tao JY, Wang SN, Zhong N, Chen Y. Association between SNPs in genes involved in folate metabolism and preterm birth risk. Genet Mol Res. 2015;14(1):850-859. Available from: https://doi.org/10.4238/2015.february.2.9
- van der Put NM, Eskes TK, Blom HJ. Is the common 677CT mutation in the methylenetetrahydrofolate reductase gene a risk factor for neural tube defects? A meta-analysis. Q J Med. 1997;90:111-115. Available from: https://doi.org/10.1093/qjmed/90.2.111
- Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137-146. Available from: https://doi.org/10.1016/s0166-2236(03)00032-8
Figures:
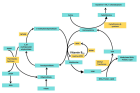
Figure 1
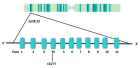
Figure 2
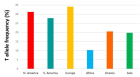
Figure 3
Figure 4
Similar Articles
-
Managing epileptic women in pregnancySarmad Muhammad Soomar*,Saima Rajpali. Managing epileptic women in pregnancy. . 2019 doi: 10.29328/journal.cjog.1001015; 2: 001-002
-
Comparative effect of calcium supplementation on the incidence of pre-eclampsia and eclampsia among primigravid womenAssontsa Kafack Carole*,Essiben Felix,Tumasang Florence,Meka Esther Juliette,Tongo Sedrick Fofack,Mbu Robinson Enow. Comparative effect of calcium supplementation on the incidence of pre-eclampsia and eclampsia among primigravid women. . 2019 doi: 10.29328/journal.cjog.1001038; 2: 145-149
-
COVID-19 in pregnancy: Our experience at a tertiary maternity unit in FranceDarido Jessie*,El Haddad Cynthia,Diari Jed,Grevoul Fesquet Julie,Bouzid Nassima,Bobric Andrea,Lakhdara Nefissa,Bazzi Zeinab,Lebis Cindy,Khadam Louay,Rigonnot Luc. COVID-19 in pregnancy: Our experience at a tertiary maternity unit in France. . 2020 doi: 10.29328/journal.cjog.1001051; 3: 054-064
-
Pregnancy complicated with deficiency of antithrombin: Review of current literatureMiroslava Gojnic,Zoran Vilendecic,Stefan Dugalic,Igor Pantic,Jovana Todorovic,Milan Perovic,Mirjana Kovac,Irena Djunic,Predrag Miljic,Jelena Dotlic*. Pregnancy complicated with deficiency of antithrombin: Review of current literature. . 2020 doi: 10.29328/journal.cjog.1001059; 3: 103-108
-
Predominance of fetal malformations among pregnant women: A multi-centric observational studySayed Mohamed Sayed,Ahmed Khedr Khalifa,Nesreen Abd El Fattah Abd Allah Shehata*,Mohamed AM Eweis,Sherwet M Shawky. Predominance of fetal malformations among pregnant women: A multi-centric observational study. . 2021 doi: 10.29328/journal.cjog.1001087; 4: 055-059
-
A Genetic study in assisted reproduction and the risk of congenital anomaliesKaparelioti Chrysoula,Koniari Eleni*,Efthymiou Vasiliki,Loutradis Dimitrios,Chrousos George,Fryssira Eleni. A Genetic study in assisted reproduction and the risk of congenital anomalies. . 2021 doi: 10.29328/journal.cjog.1001095; 4: 096-100
-
Severe preeclampsia at the University Hospital Center of Mother and Child (UHCMC) in N’djamena: Epidemiology and prognosisFoumsou L*,Kouamé A,Danmadji NL,Gabkika BM,Damthéou S,Aché H. Severe preeclampsia at the University Hospital Center of Mother and Child (UHCMC) in N’djamena: Epidemiology and prognosis. . 2022 doi: 10.29328/journal.cjog.1001099; 5: 009-012
-
To compare serum Vitamin D status in pre-eclamptic and non-preeclamptic pregnant women in labour: A tertiary care centre study of Northern IndiaMonica Karpa,Sita Thakur,Kamal Singh*,Jyoti Sharma,Harsha Chaudhary. To compare serum Vitamin D status in pre-eclamptic and non-preeclamptic pregnant women in labour: A tertiary care centre study of Northern India. . 2022 doi: 10.29328/journal.cjog.1001100; 5: 013-018
-
Impact of various PCOS phenotypes on oocyte competence in an ART cycleNamita Jain*,Sonia Malik,Ved Prakash. Impact of various PCOS phenotypes on oocyte competence in an ART cycle. . 2022 doi: 10.29328/journal.cjog.1001110; 5: 067-071
-
Racial and Ethnic Disparities in Pregnancy-related Complications: Findings at Mansa General Hospital and 2nd Affiliated Hospital of Nanjing Medical UniversityKasonde Chanda, Liang Sheng Lian, Kong Yi Yan, Huang Qian, Gulidiya Abulikem, Royd Nkalamo Nonde, Ying Xiao Yan*. Racial and Ethnic Disparities in Pregnancy-related Complications: Findings at Mansa General Hospital and 2nd Affiliated Hospital of Nanjing Medical University. . 2023 doi: 10.29328/journal.cjog.1001131; 6: 065-075
Recently Viewed
-
Clinical and Histopathological Mismatch: A Case Report of Acral FibromyxomaMonica Mishra*,Kailas Mulsange,Gunvanti Rathod,Deepthi Konda. Clinical and Histopathological Mismatch: A Case Report of Acral Fibromyxoma. Arch Pathol Clin Res. 2025: doi: 10.29328/journal.apcr.1001045; 9: 005-007
-
Unconventional powder method is a useful technique to determine the latent fingerprint impressionsHarshita Niranjan,Shweta Rai,Kapil Raikwar,Chanchal Kamle,Rakesh Mia*. Unconventional powder method is a useful technique to determine the latent fingerprint impressions. J Forensic Sci Res. 2022: doi: 10.29328/journal.jfsr.1001035; 6: 045-048
-
Doppler Evaluation of Renal Vessels in Pediatric Patients with Relapse and Remission in Different Categories of Nephrotic SyndromeAmit Nandan Dhar Dwivedi*, Srishti Sharma, OP Mishra, Girish Singh. Doppler Evaluation of Renal Vessels in Pediatric Patients with Relapse and Remission in Different Categories of Nephrotic Syndrome. J Clini Nephrol. 2023: doi: 10.29328/journal.jcn.1001112; 7: 067-072
-
Atlantoaxial subluxation in the pediatric patient: Case series and literature reviewCatherine A Mazzola*,Catherine Christie,Isabel A Snee,Hamail Iqbal. Atlantoaxial subluxation in the pediatric patient: Case series and literature review. J Neurosci Neurol Disord. 2020: doi: 10.29328/journal.jnnd.1001037; 4: 069-074
-
Intelligent Design of Ecological Furniture in Risk Areas based on Artificial SimulationTorres del Salto Rommy Adelfa*, Bryan Alfonso Colorado Pástor*. Intelligent Design of Ecological Furniture in Risk Areas based on Artificial Simulation. Arch Surg Clin Res. 2024: doi: 10.29328/journal.ascr.1001083; 8: 062-068
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."