Abstract
Research Article
Addition of dydrogesterone to vaginal progesterone and transfer postponement improve outcomes in patients with low progesterone levels in hormonally substituted cycles for frozen-thawed embryo transfer
Anne Lecourt, Julie Labrosse, Maeliss Peigné, Claire Vinolas, Laetitia Laup, Christophe Sifer, Michael Grynberg and Isabelle Cedrin-Durnerin*
Published: 11 March, 2022 | Volume 5 - Issue 1 | Pages: 027-035
Purpose: Adding dydrogesterone (DYD) to vaginal micronized progesterone (VMP) and postponing embryo transfer in order to improve outcomes in patients with low progesterone (P) levels in hormonally substituted cycles prior to frozen/thawed embryo transfer (FET).
Methods: Endometrial preparation comprised sequential administration of vaginal estradiol until endometrial thickness reached 7 mm, followed by transdermal estradiol combined with 800 mg/day VMP. Our previous analysis of serum P levels on FET day showed that the optimal P level was > 11 ng/mL for live birth. Serum P was measured on day1 (D1) following exogenous VMP introduction in the evening. When P levels were > 11 ng/mL, FET was performed “in phase” on day-2, day-3, or day-5 depending on embryo stage at cryopreservation (n = 139 cycles). When P levels were < 11 ng/mL, DYD 10 mg three times a day orally, was added to VMP and FET was postponed by one day (n = 237 cycles, 63%). The primary endpoint was the comparison of live birth rates (LBR) between the two groups.
Results: Mean serum P level on D1 was 10.2 + 3.7 ng/mL. Characteristics of patients in both groups were similar for age, body mass index, endometrial thickness prior to P introduction, quality of transferred embryos, and embryo transfer stage. Regarding the primary endpoint, LBR was similar between the VMP+DYD group and the VMP group (26.1% vs. 27.3%, NS).
Conclusion: These results suggest that adding DYD to VMP and postponing the transfer in patients with low P levels in hormonally substituted FET cycles might optimize outcomes.
Read Full Article HTML DOI: 10.29328/journal.cjog.1001103 Cite this Article Read Full Article PDF
Keywords:
Frozen thawed embryo transfer; Hormone replacement treatment; Serum progesterone; Dydrogesterone; Live birth
References
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017; 23: 139-155. PubMed: https://pubmed.ncbi.nlm.nih.gov/27827818/
- Belva F, Bonduelle M, Roelants M, Verheyen G, Van Landuyt L. Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Hum Reprod. 2016; 31: 1610–1620. PubMed: https://pubmed.ncbi.nlm.nih.gov/27165622/
- Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013; 19: 433–457. PubMed: https://pubmed.ncbi.nlm.nih.gov/23827986/
- Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Gynaecology and Fertility Group, editor. Cochrane Database Syst Rev. 2017; 7: CD003414.PubMed: https://pubmed.ncbi.nlm.nih.gov/28675921/
- Groenewoud ER, Cohlen BJ, Macklon NS. Programming the endometrium for deferred transfer of cryopreserved embryos: hormone replacement versus modified natural cycles. Fertil Steril. 2018; 109: 768–774. PubMed: https://pubmed.ncbi.nlm.nih.gov/29778369/
- Yarali H, Polat M, Mumusoglu S, Yarali I, Bozdag G. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta-analysis. J Assist Reprod Genet. 2016; 33: 1287–1304. PubMed: https://pubmed.ncbi.nlm.nih.gov/27549760/
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, et al. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Gynaecology and Fertility Group, editor. Cochrane Database Syst Rev. 2020; 10: CD006359.PubMed: https://pubmed.ncbi.nlm.nih.gov/20091592/
- Kahraman S, Karagozoglu SH, Karlıkaya G. The efficiency of progesterone vaginal gel versus intramuscular progesterone for luteal phase supplementation in gonadotropin-releasing hormone antagonist cycles: a prospective clinical trial. Fertil Steril. 2010; 94: 761–763. PubMed: https://pubmed.ncbi.nlm.nih.gov/19939363/
- Polat M, Mumusoglu S, Bozdag G, Ozbek IY, Humaidan P, et al. Addition of intramuscular progesterone to vaginal progesterone in hormone replacement therapy in vitrified–warmed blastocyst transfer cycles. Reprod Biomed Online. 2020; 40: 812–818. PubMed: https://pubmed.ncbi.nlm.nih.gov/32362573/
- Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP. Progesterone replacement with vaginal gel versus i.m. injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod. 2014; 29: 1706–1711. PubMed: https://pubmed.ncbi.nlm.nih.gov/24847018/
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization–embryo transfer cycles: a prospective randomized study. Fertil Steril. 2010; 94: 2596–2599. PubMed: https://pubmed.ncbi.nlm.nih.gov/20347079/
- Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, et al. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study**Presented at the 40th Annual Meeting of the Pacific Coast Fertility Society, Indian Wells, California, April 8 to 12, 1992. Fertil Steril. 1994; 62: 485–490. PubMed: https://pubmed.ncbi.nlm.nih.gov/8062942/
- Nahoul K, Dehennin L, Jondet M, Roger M. Profiles of plasma estrogens, progesterone and their metabolites after oral or vaginal administration of estradiol or progesterone. Maturitas. 1993; 16: 185–202. PubMed: https://pubmed.ncbi.nlm.nih.gov/8515718/
- Paulson RJ, Collins MG, Yankov VI. Progesterone Pharmacokinetics and Pharmacodynamics With 3 Dosages and 2 Regimens of an Effervescent Micronized Progesterone Vaginal Insert. J Clin Endocrinol Metab. 2014; 99: 4241–4249. PubMed: https://pubmed.ncbi.nlm.nih.gov/24606090/
- Duijkers IJM, Klingmann I, Prinz R, Wargenau M, Hrafnsdottir S, et al. Effect on endometrial histology and pharmacokinetics of different dose regimens of progesterone vaginal pessaries, in comparison with progesterone vaginal gel and placebo. Hum Reprod. 2018; 33: 2131-2140. PubMed: https://pubmed.ncbi.nlm.nih.gov/30265306/
- Yovich JL, Conceicao JL, Stanger JD, Hinchliffe PM, Keane KN. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reprod Biomed Online. 2015; 31: 180–191. PubMed: https://pubmed.ncbi.nlm.nih.gov/26099447/
- Labarta E, Mariani G, Holtmann N, Celada P, Remohí J, et al. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod. 2017; 32: 2437–2442. PubMed: https://pubmed.ncbi.nlm.nih.gov/29040638/
- Gaggiotti-Marre S, Martinez F, Coll L, Garcia S, Álvarez M, et al. Low serum progesterone the day prior to frozen embryo transfer of euploid embryos is associated with significant reduction in live birth rates. Gynecol Endocrinol. 2019; 35: 439–442. PubMed: https://pubmed.ncbi.nlm.nih.gov/30585507/
- Cédrin-Durnerin I, Isnard T, Mahdjoub S, Sonigo C, Seroka A, et al. Serum progesterone concentration and live birth rate in frozen–thawed embryo transfers with hormonally prepared endometrium. Reprod Biomed Online. 2019; 38: 472–480. PubMed: https://pubmed.ncbi.nlm.nih.gov/30642638/
- Labarta E, Mariani G, Paolelli S, Rodriguez-Varela C, Vidal C, et al. Impact of low serum progesterone levels on the day of embryo transfer on pregnancy outcome: a prospective cohort study in artificial cycles with vaginal progesterone. Hum Reprod. 2021; 36: 683–692. PubMed: https://pubmed.ncbi.nlm.nih.gov/33340402/
- Maignien C, Bourdon M, Marcellin L, Laguillier-Morizot C, Borderie D, et al. Low serum progesterone affects live birth rate in cryopreserved blastocyst transfer cycles using hormone replacement therapy. Reprod Biomed Online. 2021; S1472-6483(21)00579-4. PubMed: https://pubmed.ncbi.nlm.nih.gov/34980570/
- Basnayake SK, Volovsky M, Rombauts L, Osianlis T, Vollenhoven B, et al. Progesterone concentrations and dosage with frozen embryo transfers - What’s best? Aust N Z J Obstet Gynaecol. 2018; 58: 533–538. PubMed: https://pubmed.ncbi.nlm.nih.gov/29271471/
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, et al. Progesterone levels on pregnancy test day after hormone replacement therapy-cryopreserved embryo transfer cycles and related reproductive outcomes. Reprod Biomed Online. 2018; 37: 641–647. PubMed: https://pubmed.ncbi.nlm.nih.gov/30385142/
- Commissaire M, Epelboin S, Vigan M, Tubiana S, Llabador MA, et al. Serum progesterone level and ongoing pregnancy rate following frozen-thawed embryo transfer after artificial endometrial preparation: a monocentric retrospective study. J Gynecol Obstet Hum Reprod. 2020; 49: 101828. PubMed: https://pubmed.ncbi.nlm.nih.gov/32534215/
- Labarta E, Rodriguez-Varela C, Mariani G, Bosch E. Serum progesterone profile across the mid and late luteal phase in artificial cycles is associated with pregnancy outcome. Front. Endocrinol. 2021; 12: 665717. PubMed: https://pubmed.ncbi.nlm.nih.gov/34177806/
- González-Foruria I, Gaggiotti-Marre S, Álvarez M, Martínez F, García S, et al. Factors associated with serum progesterone concentrations the day before cryopreserved embryo transfer in artificial cycles. Reprod Biomed Online. 2020; 40: 797–804. PubMed: https://pubmed.ncbi.nlm.nih.gov/32386938/
- Labrosse J, Peigné M, Eustache F, Sifer C, Grynberg M, et al. Women utilizing oocyte donation have a decreased live birth rate if they displayed a low progesterone level in a previous hormonal replacement mock cycle. J Assist Reprod Genet. 2021; 38: 605–612. PubMed: https://pubmed.ncbi.nlm.nih.gov/33415529/
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, et al. Can combining vaginal and rectal progesterone achieve the optimum progesterone range required for implantation in the HRT-FET model? Reprod Biomed Online. 2020; 40: 805–811. PubMed: https://pubmed.ncbi.nlm.nih.gov/32376312/
- Labarta E, Mariani G, Rodríguez-Varela C, Bosch E. Individualized luteal phase support normalizes live birth rate in women with low progesterone levels on the day of embryo transfer in artificial endometrial preparation cycles. Fertil Steril. 2022; 117: 96-103. PubMed: https://pubmed.ncbi.nlm.nih.gov/34548167/
- Álvarez M, Gaggiotti-Marre S, Martínez F, Coll L, García S, et al. Individualised luteal phase support in artificially prepared frozen embryo transfer cycles based on serum progesterone levels: a prospective cohort study. Hum Reprod. 2021; 36: 1552-1560. PubMed: https://pubmed.ncbi.nlm.nih.gov/33686413/
- Yarali H, Polat M, Mumusoglu S, Ozbek IY, Erden M, et al. Subcutaneous luteal phase progesterone rescue rectifies ongoing pregnancy rates in hormone replacement therapy vitrified–warmed blastocyst transfer cycles. Reprod Biomed Online. 2021; 43: 45-51. PubMed: https://pubmed.ncbi.nlm.nih.gov/34016521/
- Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, et al. Selectivity and potency of the retroprogesterone dydrogesterone in vitro. Steroids. 2011; 76: 607–615. PubMed: https://pubmed.ncbi.nlm.nih.gov/21376746/
- Stanczyk FZ, Hapgood JP, Winer S, Mishell DR. Progestogens Used in Postmenopausal Hormone Therapy: Differences in Their Pharmacological Properties, Intracellular Actions, and Clinical Effects. Endocr Rev. 2013; 34: 171–208. PubMed: https://pubmed.ncbi.nlm.nih.gov/23238854/
- Barbosa MWP, Silva LR, Navarro PA, Ferriani RA, Nastri CO, et al. Dydrogesterone vs progesterone for luteal-phase support: systematic review and meta-analysis of randomized controlled trials: Dydrogesterone for assisted reproduction. Ultrasound Obstet Gynecol. 2016; 48: 161–170. PubMed: https://pubmed.ncbi.nlm.nih.gov/26577241/
- Griesinger G, Tournaye H, Macklon N, Petraglia F, Arck P, et al. Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction. Reprod Biomed Online. 2019; 38: 249–259. PubMed: https://pubmed.ncbi.nlm.nih.gov/30595525/
- Griesinger G, Blockeel C, T. Sukhikh G, Patki A, Dhorepatil B, et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial. Hum Reprod. 2018; 33: 2212-2221.PubMed: https://pubmed.ncbi.nlm.nih.gov/30304457/
- Tournaye H, Sukhikh GT, Kahler E, Griesinger G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017; 32: 1019–1027. PubMed: https://pubmed.ncbi.nlm.nih.gov/28333318/
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Gynaecology and Fertility Group, editor. Cochrane Database Syst Rev. 2015: CD009154.PubMed: https://pubmed.ncbi.nlm.nih.gov/26148507/
- Rashidi BH, Ghazizadeh M, Tehrani Nejad ES, Bagheri M, Gorginzadeh M. Oral dydrogesterone for luteal support in frozen-thawed embryo transfer artificial cycles: A pilot randomized controlled trial. Asian Pac J Reprod. 2016; 5: 490–494.
- Zarei A, Sohail P, Parsanezhad ME, Alborzi S, Samsami A, et al. Comparison of four protocols for luteal phase support in frozen-thawed Embryo transfer cycles: a randomized clinical trial. Arch Gynecol Obstet. 2017; 295: 239–246. PubMed: https://pubmed.ncbi.nlm.nih.gov/27761732/
- Holte J, Berglund L, Milton K, Garello C, Gennarelli G, et al. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007; 22: 548–557. PubMed: https://pubmed.ncbi.nlm.nih.gov/17095516/
- Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts: Curr Opin Obstet Gynaecol. 1999; 11: 307–311. PubMed: https://pubmed.ncbi.nlm.nih.gov/10369209/
- Volovsky M, Pakes C, Rozen G, Polyakov A. Do serum progesterone levels on day of embryo transfer influence pregnancy outcomes in artificial frozen-thaw cycles? J Assist Reprod Genet. 2020; 37: 1129–1135. PubMed: https://pubmed.ncbi.nlm.nih.gov/32043182/
- Boynukalin FK, Gultomruk M, Cavkaytar S, Turgut E, Findikli N, et al. Parameters impacting the live birth rate per transfer after frozen single euploid blastocyst transfer. Viganò P, editor. PLOS ONE. 2020; 15: e0227619. PubMed: https://pubmed.ncbi.nlm.nih.gov/31929583/
- Delcour C, Robin G, Delesalle AS, Drumez E, Plouvier P, et al. Weekly intramuscular progesterone for luteal phase support in women receiving oocyte donation is associated with a decreased miscarriage rate. Reprod Biomed Online. 2019; 39: 446–451. PubMed: https://pubmed.ncbi.nlm.nih.gov/31311693/
- Devine K, Richter KS, Jahandideh S, Widra EA, McKeeby JL. Intramuscular progesterone optimizes live birth from programmed frozen embryo transfer: a randomized clinical trial. Fertil Steril. 2021; 116: 633-643. PubMed: https://pubmed.ncbi.nlm.nih.gov/33992421/
- Ramos NN, Pirtea P, Benammar A, Ziegler D de, Jolly E, et al. Is there a link between plasma progesterone 1–2 days before frozen embryo transfers (FET) and ART outcomes in frozen blastocyst transfers? Gynecol Endocrinol. 2020; 1–4. PubMed: https://pubmed.ncbi.nlm.nih.gov/32996332/
- Alyasin A, Agha-Hosseini M, Kabirinasab M, Saeidi H, Nashtaei MS. Serum progesterone levels greater than 32.5 ng/mL on the day of embryo transfer are associated with lower live birth rate after artificial endometrial preparation: a prospective study. Reprod Biol Endocrinol. 2021; 19: 24. PubMed: https://pubmed.ncbi.nlm.nih.gov/33602270/
- Abdel-Hamid ME, Sharaf LH, Kombian SB, Diejomaoh FME. Determination of Dydrogesterone in Human Plasma by Tandem Mass Spectrometry: Application to Therapeutic Drug Monitoring of Dydrogesterone in Gynecological Disorders. Chromatographia. 2006; 64: 287–292. PubMed: https://pubag.nal.usda.gov/catalog/4392298
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, et al. Classification and pharmacology of progestins. Maturitas. 2003; 46: 7–16. PubMed: https://pubmed.ncbi.nlm.nih.gov/14670641/
- Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, et al. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: Results of a randomised study. J Steroid Biochem Mol Biol. 2005; 97: 416–420. PubMed: https://pubmed.ncbi.nlm.nih.gov/16213136/
- Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015; 186: 49–53. PubMed: https://pubmed.ncbi.nlm.nih.gov/25622239/
- Zaqout M, Aslem E, Abuqamar M, Abughazza O, Panzer J, et al. The Impact of Oral Intake of Dydrogesterone on Fetal Heart Development During Early Pregnancy. Pediatr Cardiol. 2015; 36: 1483–1488. PubMed: https://pubmed.ncbi.nlm.nih.gov/25972284/
- Vuong LN, Pham TD, Le KTQ, Ly TT, Le HL, et al. Micronized progesterone plus dydrogesterone versus micronized progesterone alone for luteal phase support in frozen-thawed cycles (MIDRONE): a prospective cohort study. Hum Reprod. 2021; 36: 1821–1831. PubMed: https://pubmed.ncbi.nlm.nih.gov/33930124/
Figures:

Figure 1
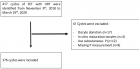
Figure 2
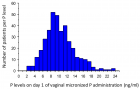
Figure 3
Similar Articles
-
The validity of progesterone level on hCG injection day in the prediction of IVF/ICSI cycles’ outcomeMostafa A Abolfotouh*,Hallah Al-Anazi,Samar Hassan. The validity of progesterone level on hCG injection day in the prediction of IVF/ICSI cycles’ outcome. . 2019 doi: 10.29328/journal.cjog.1001028; 2: 095-100
-
Addition of dydrogesterone to vaginal progesterone and transfer postponement improve outcomes in patients with low progesterone levels in hormonally substituted cycles for frozen-thawed embryo transferAnne Lecourt,Julie Labrosse,Maeliss Peigné,Claire Vinolas,Laetitia Laup,Christophe Sifer,Michael Grynberg,Isabelle Cedrin-Durnerin*. Addition of dydrogesterone to vaginal progesterone and transfer postponement improve outcomes in patients with low progesterone levels in hormonally substituted cycles for frozen-thawed embryo transfer. . 2022 doi: 10.29328/journal.cjog.1001103; 5: 027-035
-
Neonatal Mortality Rate among Twin and Singleton Births with the Gestational Age of 34-37 Weeks: A Population-Based StudySedigheh Hantoushzadeh, Kayvan Mirnia, Hananeh Sadat Sadeghi, Parvaneh Sadeghimoghadam*, Mohammad Aghaali, Mohammad Heidarzadeh, Abbas Habibelahi, Shima Rafiee, Mohammad Haddadi, Amir Naddaf. Neonatal Mortality Rate among Twin and Singleton Births with the Gestational Age of 34-37 Weeks: A Population-Based Study. . 2023 doi: 10.29328/journal.cjog.1001134; 6: 088-094
-
Near-miss Women Causes and Prevalence in Alobied Maternity HospitalAyat Eltigani, Taha Umbeli Ahmed, Awadalla Abdelwahid Suliman*, Abdelsalam SalahEldin, Isra Siralkatim, Hajar Suliman. Near-miss Women Causes and Prevalence in Alobied Maternity Hospital. . 2023 doi: 10.29328/journal.cjog.1001149; 6: 185-192
-
COVID-19 Pneumonia in Pregnancy: A Retrospective Study on Maternal and Neonatal OutcomesBenlghazi Abdelhamid*, Belouad Moad, Hanane Dabdi, Bouhtouri Yassine, Messaoudi Hamza1, Benali Saad, Ait Bouhou Rachid, El Mangoub Fatima, Elhassani Mly El Mehdi, Kouach Jaouad. COVID-19 Pneumonia in Pregnancy: A Retrospective Study on Maternal and Neonatal Outcomes. . 2024 doi: 10.29328/journal.cjog.1001163; 7: 051-055
-
Prospective Community-based Study of Still Births in Remote Villages with Low ResourcesShakuntala Chhabra*, Naman Chhabda, Afreen S, Rathod M. Prospective Community-based Study of Still Births in Remote Villages with Low Resources. . 2024 doi: 10.29328/journal.cjog.1001168; 7: 072-077
-
The Clinical Pregnancy and Live Birth Following Transfer of One Arrested Embryo: A Case ReportAli Asghar Ghafarizade, Elham Shojafar*, Samira Naderi, Fatemeh Seifi, Alireza Noshad, Zohreh Lavasani, Zahra Kalhori, Elahe Ghadiri. The Clinical Pregnancy and Live Birth Following Transfer of One Arrested Embryo: A Case Report. . 2024 doi: 10.29328/journal.cjog.1001175; 7: 112-114
Recently Viewed
-
Gilbert’s Syndrome Revealed by Hepatotoxicity of ImatinibImen Ben Amor*,Imen Frikha,Moez Medhaffer,Moez Elloumi. Gilbert’s Syndrome Revealed by Hepatotoxicity of Imatinib. Ann Clin Gastroenterol Hepatol. 2025: doi: 10.29328/journal.acgh.1001049; 9: 001-003
-
The pathogenesis of psoriasis: insight into a complex “Mobius Loop” regulation processYuankuan Jiang,Haiyang Chen,Jiayue Liu,Tianfu Wei,Peng Ge,Jialin Qu*,Jingrong Lin. The pathogenesis of psoriasis: insight into a complex “Mobius Loop” regulation process. Arch Pathol Clin Res. 2021: doi: 10.29328/journal.apcr.1001024; 5: 020-025
-
Potential Use of Essential Oils and Their Individual Components in Cosmeceuticals: A ReviewHamdy A Shaaban*. Potential Use of Essential Oils and Their Individual Components in Cosmeceuticals: A Review. Ann Biomed Sci Eng. 2023: doi: 10.29328/journal.abse.1001023; 7: 031-037
-
Studies on some spices and herbs: Chemical composition, health benefits and functional propertiesHamdy A Shaaban*. Studies on some spices and herbs: Chemical composition, health benefits and functional properties. Ann Biomed Sci Eng. 2023: doi: 10.29328/journal.abse.1001019; 7: 001-011
-
About Efficiency of High-order Harmonic Generation in Attosecond PhysicsAng-Yang Yu*. About Efficiency of High-order Harmonic Generation in Attosecond Physics. Int J Clin Virol. 2024: doi: 10.29328/journal.ijcv.1001061; 8: 045-047
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."